当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calcium–MicroRNA Complex-Functionalized Nanotubular Implant Surface for Highly Efficient Transfection and Enhanced Osteogenesis of Mesenchymal Stem Cells
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsami.7b18289
Wen Song 1 , Chuanxu Yang , Dang Quang Svend Le 2 , Yumei Zhang 1 , Jørgen Kjems
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsami.7b18289
Wen Song 1 , Chuanxu Yang , Dang Quang Svend Le 2 , Yumei Zhang 1 , Jørgen Kjems
Affiliation
|
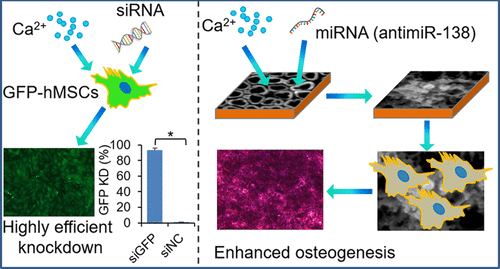
Controlling mesenchymal stem cell (MSC) differentiation by RNA interference (RNAi) is a promising approach for next-generation regenerative medicine. However, efficient delivery of RNAi therapeutics is still a limiting factor. In this study, we have developed a simple, biocompatible, and highly effective delivery method of small RNA therapeutics into human MSCs (hMSCs) from an implant surface by calcium ions. First, we demonstrated that simple Ca/siRNA targeting green fluorescent protein (GFP) nanocomplexes were able to efficiently silence GFP in GFP-expressing hMSCs with adequate Ca2+ concentration (>5 mM). In addition, a single transfection could obtain a long-lasting silencing effect for more than 2 weeks. All three of the main endocytosis pathways (clathrin- and caveolin-mediated endocytosis and macropinocytosis) were involved in the internalization of the Ca/siRNA complexes by MSCs, and macropinocytosis plays the most dominant role. Furthermore, the Ca/siRNA complexes could be efficiently loaded onto the titanium implant surface when pretreated with anodization to create a nanotube (NT) layer. Because of the hydrophilic property of the NT surface, the Ca/siRNA was quickly loaded (less than 4 h) with high efficiency (nearly 100%), forming an even amorphous coating. The Ca/siRNA-coated NT surface showed an initial burst release of 80% of the siRNA complexes over 2 h, which is adequate to achieve robust gene silencing of attached hMSCs. To demonstrate the therapeutic potential of our Ca/siRNA coating technology, Ca/antimiR-138 complexes were loaded on to the NT surface, which strongly enhanced the osteogenic differentiation of hMSCs. In conclusion, our findings suggest that Ca2+ is an effective and biocompatible carrier to deliver small RNA therapeutics into hMSCs, both in solution and from functionalized surfaces, which provides a novel approach to control the MSC differentiation and tissue regeneration.
中文翻译:

钙-MicroRNA复杂功能的纳米管植入物表面的高效转染和增强间充质干细胞成骨。
通过RNA干扰(RNAi)控制间充质干细胞(MSC)分化是下一代再生医学的有前途的方法。然而,RNAi治疗剂的有效递送仍然是限制因素。在这项研究中,我们已经开发出一种简单,生物相容且高效的小钙治疗剂通过钙离子从植入物表面植入人MSC(hMSC)的方法。首先,我们证明了靶向Ca / siRNA的绿色荧光蛋白(GFP)纳米复合物能够有效沉默具有足够Ca 2+的GFP表达hMSC中的GFP。浓度(> 5 mM)。此外,单次转染可获得超过2周的长效沉默效果。所有三个主要的内吞途径(clathrin和小窝蛋白介导的内吞作用和大胞饮作用)都参与了MSC对Ca / siRNA复合物的内在化作用,而大胞饮作用起着最主要的作用。此外,在进行阳极氧化预处理以形成纳米管(NT)层时,可以将Ca / siRNA复合物有效地负载到钛植入物表面上。由于NT表面具有亲水性,因此Ca / siRNA可以高效(几乎100%)快速加载(少于4小时),从而形成均匀的无定形涂层。Ca / siRNA包被的NT表面在2小时内显示出80%的siRNA复合物的初始爆发释放,这足以实现对附着的hMSC的强大基因沉默。为了证明我们的Ca / siRNA包被技术的治疗潜力,将Ca / antimiR-138复合物加载到NT表面,这大大增强了hMSC的成骨分化能力。总之,我们的发现表明钙2+是一种有效的生物相容性载体,可将小分子RNA治疗剂从溶液中和功能化表面递送到hMSC中,这为控制MSC分化和组织再生提供了一种新颖的方法。
更新日期:2018-02-08
中文翻译:

钙-MicroRNA复杂功能的纳米管植入物表面的高效转染和增强间充质干细胞成骨。
通过RNA干扰(RNAi)控制间充质干细胞(MSC)分化是下一代再生医学的有前途的方法。然而,RNAi治疗剂的有效递送仍然是限制因素。在这项研究中,我们已经开发出一种简单,生物相容且高效的小钙治疗剂通过钙离子从植入物表面植入人MSC(hMSC)的方法。首先,我们证明了靶向Ca / siRNA的绿色荧光蛋白(GFP)纳米复合物能够有效沉默具有足够Ca 2+的GFP表达hMSC中的GFP。浓度(> 5 mM)。此外,单次转染可获得超过2周的长效沉默效果。所有三个主要的内吞途径(clathrin和小窝蛋白介导的内吞作用和大胞饮作用)都参与了MSC对Ca / siRNA复合物的内在化作用,而大胞饮作用起着最主要的作用。此外,在进行阳极氧化预处理以形成纳米管(NT)层时,可以将Ca / siRNA复合物有效地负载到钛植入物表面上。由于NT表面具有亲水性,因此Ca / siRNA可以高效(几乎100%)快速加载(少于4小时),从而形成均匀的无定形涂层。Ca / siRNA包被的NT表面在2小时内显示出80%的siRNA复合物的初始爆发释放,这足以实现对附着的hMSC的强大基因沉默。为了证明我们的Ca / siRNA包被技术的治疗潜力,将Ca / antimiR-138复合物加载到NT表面,这大大增强了hMSC的成骨分化能力。总之,我们的发现表明钙2+是一种有效的生物相容性载体,可将小分子RNA治疗剂从溶液中和功能化表面递送到hMSC中,这为控制MSC分化和组织再生提供了一种新颖的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号