当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spherical Closo Deltahedra with Surface Metal–Metal Multiple Bonding versus Oblate Deltahedra with Internal Metal–Metal Bonding in Dichromadicarbaborane Structures: The Nature of Stone’s Icosahedral Dichromadicarbaborane
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-01 00:00:00 , DOI: 10.1021/acs.inorgchem.8b03476 Szabolcs Jákó , Alexandru Lupan , Attila-Zsolt Kun , R. Bruce King 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-01 00:00:00 , DOI: 10.1021/acs.inorgchem.8b03476 Szabolcs Jákó , Alexandru Lupan , Attila-Zsolt Kun , R. Bruce King 1
Affiliation
|
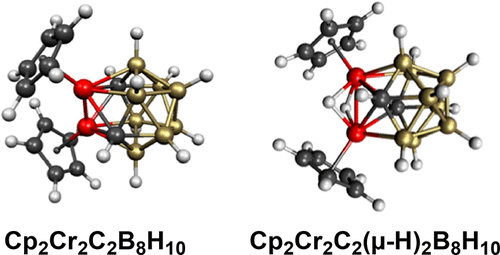
The dichromadicarbaboranes Cp2Cr2C2Bn–4Hn–2 (n = 8–12) related to the icosahedral structure reported in 1983 by Stone and co-workers and formulated by them as Cp2Cr2C2B8H10 have been investigated using density functional theory. In most cases, the lowest-energy structures are flattened oblatocloso structures with degree 6 and 7 chromium vertices similar to the lowest-energy and experimental structures of the isoelectronic dirhenaboranes Cp2Re2Bn–2Hn–2. However, most isomeric spherical closo deltahedral structures with surface Cr≣Cr quadruple bonds as well as isocloso structures with surface metal–metal Cr≡Cr triple bonds lie at accessible energies, typically lower than those in the corresponding dirhenaborane systems. However, for the 11-vertex Cp2Cr2C2B7H9 system, the most spherical closo/isocloso deltahedral structure with a degree 6 metal vertex and degree 4 carbon vertices as well as a surface M≡M triple bond lies energetically below the lowest-energy oblatocloso structure. Calculations of the Cr–Cr distances in an icosahedral Cp2Cr2C2B8H10 structure and in a dihydrogenated icosahedral Cp2Cr2(μ-H)2C2B8H10 structure suggest the latter structure for “Cp2Cr2C2B8H10” reported by Stone and co-workers.
中文翻译:

具有双金属双键键合的球形Closo Deltahedra与具有双金属双键构型的带有内部金属-金属键的扁球状的Deltahedra:石头的二十面体双金属双键的性质
Stone和他的同事在1983年报道了与二十面体结构有关的重铬双碳硼烷Cp 2 Cr 2 C 2 B n –4 H n –2(n = 8–12),他们将其配制为Cp 2 Cr 2 C 2 B 8已经使用密度泛函理论研究了H 10。在大多数情况下,最低能级结构是具有6和7级铬顶点的扁平扁豆双氧体结构,类似于等电子二烯六硼醚Cp 2 Re 2 B的最低能级和实验结构。n –2 H n –2。然而,大多数的异构体球形闭合碳deltahedral与表面Cr≣Cr四重键以及结构isocloso与表面金属-金属Cr≡Cr三键结构位于在可访问的能量,典型地比在对应dirhenaborane系统降低。然而,对于11-顶点的Cp 2的Cr 2 c ^ 2乙7 ħ 9的系统,所述最球形闭合碳/ isocloso deltahedral具有一定程度的6金属顶点和度4个碳的顶点,以及一个表面M≡M三键位于大力结构低于最低能量的oblotocloso结构体。在二十面体Cp 2 Cr 2 C 2 B 8 H 10结构和二氢化二十面体Cp 2 Cr 2(μ-H)2 C 2 B 8 H 10结构中Cr-Cr距离的计算表明,对于“ Cp Stone和同事报告了2 Cr 2 C 2 B 8 H 10 ”。
更新日期:2019-03-01
中文翻译:

具有双金属双键键合的球形Closo Deltahedra与具有双金属双键构型的带有内部金属-金属键的扁球状的Deltahedra:石头的二十面体双金属双键的性质
Stone和他的同事在1983年报道了与二十面体结构有关的重铬双碳硼烷Cp 2 Cr 2 C 2 B n –4 H n –2(n = 8–12),他们将其配制为Cp 2 Cr 2 C 2 B 8已经使用密度泛函理论研究了H 10。在大多数情况下,最低能级结构是具有6和7级铬顶点的扁平扁豆双氧体结构,类似于等电子二烯六硼醚Cp 2 Re 2 B的最低能级和实验结构。n –2 H n –2。然而,大多数的异构体球形闭合碳deltahedral与表面Cr≣Cr四重键以及结构isocloso与表面金属-金属Cr≡Cr三键结构位于在可访问的能量,典型地比在对应dirhenaborane系统降低。然而,对于11-顶点的Cp 2的Cr 2 c ^ 2乙7 ħ 9的系统,所述最球形闭合碳/ isocloso deltahedral具有一定程度的6金属顶点和度4个碳的顶点,以及一个表面M≡M三键位于大力结构低于最低能量的oblotocloso结构体。在二十面体Cp 2 Cr 2 C 2 B 8 H 10结构和二氢化二十面体Cp 2 Cr 2(μ-H)2 C 2 B 8 H 10结构中Cr-Cr距离的计算表明,对于“ Cp Stone和同事报告了2 Cr 2 C 2 B 8 H 10 ”。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号