Structure ( IF 4.4 ) Pub Date : 2019-02-28 , DOI: 10.1016/j.str.2019.01.015
Peini Hou 1 , Chang Huang 2 , Chao-Pei Liu 2 , Na Yang 3 , Tianshu Yu 4 , Yuxin Yin 4 , Bing Zhu 1 , Rui-Ming Xu 1
|
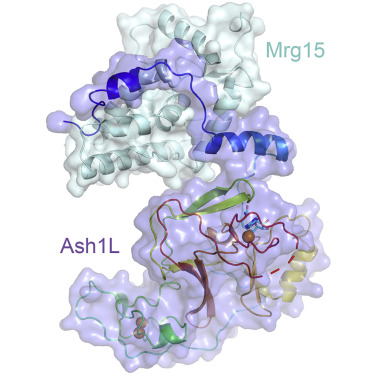
The evolutionarily conserved Trithorax group protein Ash1 is a SET domain histone methyltransferase that mono- and dimethylates lysine 36 of histone H3 (H3K36). Ash1 forms a complex with Mrg15 and Nurf55, and the binding of Mrg15 greatly stimulates the catalytic activity of Ash1, yet the underlying molecular mechanisms remain unknown. Here we report the crystal structure of the tandem Mrg15-interacting and SET domains of human Ash1L in complex with Mrg15. Ash1L interacts with Mrg15 principally via a segment located N-terminal to the catalytic SET domain. Surprisingly, an autoinhibitory loop in the post-SET region of Ash1L is destabilized on Mrg15 binding despite no direct contact. Dynamics of the autoinhibitory loop can be attributed to subtle structural changes of the S-adenosylmethionine (SAM) binding pocket induced by Mrg15 binding, implicating a mechanism of conformational coupling between SAM and substrate binding sites. The findings broaden the understanding of regulation of H3K36 methyltransferases.
中文翻译:

通过Mrg15结合刺激Ash1L的H3K36甲基转移酶活性的结构见解。
进化上保守的Trithorax组蛋白Ash1是一个SET域的组蛋白甲基转移酶,可对组蛋白H3(H3K36)的赖氨酸36单和二甲基化。Ash1与Mrg15和Nurf55形成复合物,并且Mrg15的结合极大地刺激了Ash1的催化活性,但是其潜在的分子机制仍然未知。在这里,我们报告与Mrg15复杂的人类Ash1L串联Mrg15相互作用和SET域的晶体结构。Ash1L主要通过位于催化SET结构域N端的片段与Mrg15相互作用。出人意料的是,尽管没有直接接触,Ash1L的SET后区域中的自动抑制环在Mrg15结合时不稳定。自动抑制环的动力学可归因于由Mrg15结合诱导的S-腺苷甲硫氨酸(SAM)结合口袋的细微结构变化,暗示SAM和底物结合位点之间构象偶联的机制。这些发现拓宽了对H3K36甲基转移酶调控的认识。

































 京公网安备 11010802027423号
京公网安备 11010802027423号