当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Ring‐Opening of Di‐Substituted Epoxides Catalysed by Halohydrin Dehalogenases
ChemCatChem ( IF 3.8 ) Pub Date : 2019-03-15 , DOI: 10.1002/cctc.201900103 Elia Calderini 1 , Julia Wessel 1 , Philipp Süss 2 , Patrick Schrepfer 1 , Rainer Wardenga 2 , Anett Schallmey 1
ChemCatChem ( IF 3.8 ) Pub Date : 2019-03-15 , DOI: 10.1002/cctc.201900103 Elia Calderini 1 , Julia Wessel 1 , Philipp Süss 2 , Patrick Schrepfer 1 , Rainer Wardenga 2 , Anett Schallmey 1
Affiliation
|
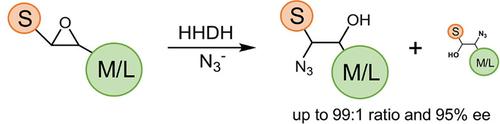
Halohydrin dehalogenases (HHDHs) are valuable biocatalysts for the synthesis of β‐substituted alcohols based on their epoxide ring‐opening activity with a number of small anionic nucleophiles. In an attempt to further broaden the scope of substrates accepted by these enzymes, a panel of 22 HHDHs was investigated in the conversion of aliphatic and aromatic vicinally di‐substituted trans‐epoxides using azide as nucleophile. The majority of these HHDHs was able to convert aliphatic methyl‐substituted epoxide substrates to the corresponding azidoalcohols, in some cases even with absolute regioselectivity. HheG from Ilumatobacter coccineus exhibited also high activity towards sterically more demanding di‐substituted epoxides. This further expands the range of β‐substituted alcohols that are accessible by HHDH catalysis.
中文翻译:

卤代醇脱卤酶催化的二取代环氧化物的选择性开环
卤代醇脱卤酶(HHDHs)是用于合成β-取代醇的有价值的生物催化剂,基于它们与许多小阴离子亲核试剂的环氧化物开环活性。在试图进一步拓宽这些酶底物接受的范围内,22个HHDHs一个面板中的脂族和芳族邻位二取代的转化进行了研究反式使用叠氮化物亲核试剂作为环氧化物。这些HHDH中的大多数能够将脂族甲基取代的环氧底物转化为相应的叠氮醇,甚至在某些情况下甚至具有绝对的区域选择性。来自球孢杆菌的HheG对空间更苛刻的二取代环氧化物也表现出高活性。这进一步扩大了HHDH催化可及的β-取代醇的范围。
更新日期:2019-03-15
中文翻译:

卤代醇脱卤酶催化的二取代环氧化物的选择性开环
卤代醇脱卤酶(HHDHs)是用于合成β-取代醇的有价值的生物催化剂,基于它们与许多小阴离子亲核试剂的环氧化物开环活性。在试图进一步拓宽这些酶底物接受的范围内,22个HHDHs一个面板中的脂族和芳族邻位二取代的转化进行了研究反式使用叠氮化物亲核试剂作为环氧化物。这些HHDH中的大多数能够将脂族甲基取代的环氧底物转化为相应的叠氮醇,甚至在某些情况下甚至具有绝对的区域选择性。来自球孢杆菌的HheG对空间更苛刻的二取代环氧化物也表现出高活性。这进一步扩大了HHDH催化可及的β-取代醇的范围。































 京公网安备 11010802027423号
京公网安备 11010802027423号