当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct access to 2-aryl substituted pyrrolinium salts for carbon centre based radicals without pyrrolidine-2-ylidene alias cyclic(alkyl)(amino)carbene (CAAC) as a precursor†‡
Chemical Science ( IF 7.6 ) Pub Date : 2019-02-28 00:00:00 , DOI: 10.1039/c8sc05477k Debdeep Mandal 1, 2, 3 , Sebastian Sobottka 4, 5, 6, 7 , Ramapada Dolai 1, 2, 3 , Avijit Maiti 1, 2, 3 , Debabrata Dhara 1, 2, 3 , Pankaj Kalita 3, 8, 9, 10, 11 , Ramakirushnan Suriya Narayanan 1, 2, 3 , Vadapalli Chandrasekhar 1, 2, 3, 12, 13 , Biprajit Sarkar 4, 5, 6, 7 , Anukul Jana 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2019-02-28 00:00:00 , DOI: 10.1039/c8sc05477k Debdeep Mandal 1, 2, 3 , Sebastian Sobottka 4, 5, 6, 7 , Ramapada Dolai 1, 2, 3 , Avijit Maiti 1, 2, 3 , Debabrata Dhara 1, 2, 3 , Pankaj Kalita 3, 8, 9, 10, 11 , Ramakirushnan Suriya Narayanan 1, 2, 3 , Vadapalli Chandrasekhar 1, 2, 3, 12, 13 , Biprajit Sarkar 4, 5, 6, 7 , Anukul Jana 1, 2, 3
Affiliation
|
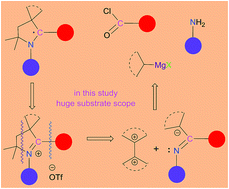
The synthesis of organic radicals is challenging due to their inherent instability. In recent years, cyclic(alkyl)(amino)carbene (CAAC)-derived 2-substituted pyrrolinium salts have been used as synthons for the synthesis of isolable carbon-based radicals. Herein, we report a direct, easy and convenient method for the synthesis of 2-aryl substituted pyrrolinium salts without using CAAC as a precursor. These cations can be reduced to the corresponding radicals. The influence of the aryl substituent at the C-2 position on radical stabilization and dimerization has been investigated. Because of the large scope of our strategy (capability to modulate different substituents at all the C- and N-centres of the pyrrolinium salts), it has the merit to be an extremely effective and productive route for generating carbon-based radicals whose stability as well as reactivity can be varied.
中文翻译:

直接获得2-芳基取代的吡咯鎓盐,可用于碳中心基团,而无需吡咯烷-2-亚烷基别名环状(烷基)(氨基)卡宾(CAAC)作为前体† ‡
有机自由基的合成由于其固有的不稳定性而具有挑战性。近年来,环状(烷基)(氨基)卡宾(CAAC)-衍生的2-取代的吡咯鎓盐已被用作合成可分离的碳基自由基的合成子。在此,我们报道了一种直接,简单,方便的方法,无需使用CAAC作为前体即可合成2-芳基取代的吡咯鎓盐。这些阳离子可以还原为相应的自由基。已经研究了在C-2位上的芳基取代基对自由基稳定和二聚作用的影响。由于我们的策略范围很广(能够调节所有C-和N上不同的取代基%的吡咯盐的优点在于,它是产生稳定性和反应性可变化的碳基自由基的极其有效和生产性的途径。
更新日期:2019-02-28
中文翻译:

直接获得2-芳基取代的吡咯鎓盐,可用于碳中心基团,而无需吡咯烷-2-亚烷基别名环状(烷基)(氨基)卡宾(CAAC)作为前体† ‡
有机自由基的合成由于其固有的不稳定性而具有挑战性。近年来,环状(烷基)(氨基)卡宾(CAAC)-衍生的2-取代的吡咯鎓盐已被用作合成可分离的碳基自由基的合成子。在此,我们报道了一种直接,简单,方便的方法,无需使用CAAC作为前体即可合成2-芳基取代的吡咯鎓盐。这些阳离子可以还原为相应的自由基。已经研究了在C-2位上的芳基取代基对自由基稳定和二聚作用的影响。由于我们的策略范围很广(能够调节所有C-和N上不同的取代基%的吡咯盐的优点在于,它是产生稳定性和反应性可变化的碳基自由基的极其有效和生产性的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号