当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective Active Chlorine Generation Electrocatalyzed by Co3O4 Nanoparticles: Mechanistic Investigation through in Situ Electrokinetic and Spectroscopic Analyses
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2019-02-28 00:00:00 , DOI: 10.1021/acs.jpclett.9b00547
Heonjin Ha 1 , Kyoungsuk Jin 1 , Sunghak Park 1 , Kang-Gyu Lee 1 , Kang Hee Cho 1 , Hongmin Seo 1 , Hyo-Yong Ahn 1 , Yoon Ho Lee 1 , Ki Tae Nam 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2019-02-28 00:00:00 , DOI: 10.1021/acs.jpclett.9b00547
Heonjin Ha 1 , Kyoungsuk Jin 1 , Sunghak Park 1 , Kang-Gyu Lee 1 , Kang Hee Cho 1 , Hongmin Seo 1 , Hyo-Yong Ahn 1 , Yoon Ho Lee 1 , Ki Tae Nam 1
Affiliation
|
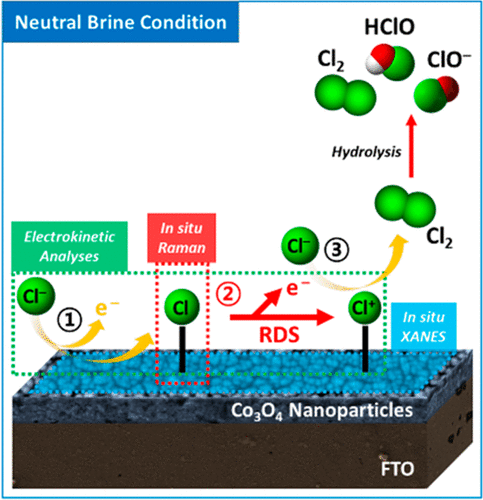
The reaction mechanism of electrochemical chloride oxidation at neutral pH is different from that at acidic pH, in which a commercial chlor-alkali process has been developed. Different proton concentrations and accelerated hydrolysis of the generated chlorine into hypochlorous acid at high pH can change the electrokinetics and stability of reaction intermediates. We have investigated a unique reaction mechanism of Co3O4 nanoparticles for chloride oxidation at neutral pH. In contrast with water oxidation, the valency of cobalt was not changed during chloride oxidation. Interestingly, a new intermediate of Co–Cl was captured spectroscopically, distinct from the reaction intermediate at acidic pH. In addition, Co3O4 nanoparticles exhibited high selectivity for active chlorine generation at neutral pH, comparable to commercially available RuO2-based catalysts. We believe that this study provides insight into designing efficient electrocatalysts for active chlorine generation at neutral pH, which can be practically applied to electrochemical water treatment coupled to hydrogen production.
中文翻译:

Co 3 O 4纳米粒子电催化的高选择性活性氯生成:通过原位电动和光谱分析的机理研究
在中性pH下电化学氯化物氧化的反应机理不同于在酸性pH下的化学机理,在酸性pH下已经开发了商业的氯碱法。在高pH下,不同的质子浓度和所生成的氯加速水解为次氯酸可改变反应中间体的电动力学和稳定性。我们研究了Co 3 O 4纳米粒子在中性pH值下氯化物氧化的独特反应机理。与水氧化相反,在氯化物氧化过程中钴的化合价没有变化。有趣的是,用分光光度法捕获了一种新的Co-Cl中间体,这与酸性pH下的反应中间体截然不同。另外,Co 3 O 4纳米颗粒对中性pH生成活性氯具有很高的选择性,可与市售的RuO 2基催化剂相比。我们认为,这项研究为设计有效的电催化剂以在中性pH值下生成活性氯提供了见识,该催化剂可实际应用于与制氢相关的电化学水处理。
更新日期:2019-02-28
中文翻译:

Co 3 O 4纳米粒子电催化的高选择性活性氯生成:通过原位电动和光谱分析的机理研究
在中性pH下电化学氯化物氧化的反应机理不同于在酸性pH下的化学机理,在酸性pH下已经开发了商业的氯碱法。在高pH下,不同的质子浓度和所生成的氯加速水解为次氯酸可改变反应中间体的电动力学和稳定性。我们研究了Co 3 O 4纳米粒子在中性pH值下氯化物氧化的独特反应机理。与水氧化相反,在氯化物氧化过程中钴的化合价没有变化。有趣的是,用分光光度法捕获了一种新的Co-Cl中间体,这与酸性pH下的反应中间体截然不同。另外,Co 3 O 4纳米颗粒对中性pH生成活性氯具有很高的选择性,可与市售的RuO 2基催化剂相比。我们认为,这项研究为设计有效的电催化剂以在中性pH值下生成活性氯提供了见识,该催化剂可实际应用于与制氢相关的电化学水处理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号