Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MDM2 promotes genome instability by ubiquitinating the transcription factor HBP1.
Oncogene ( IF 6.9 ) Pub Date : 2019-02-28 , DOI: 10.1038/s41388-019-0761-2
Zhengyi Cao 1 , Junhui Xue 1 , Yuning Cheng 1 , Jiyin Wang 1 , Yujuan Liu 1 , Hui Li 1 , Wei Jiang 1 , Gang Li 1 , Yaoting Gui 2 , Xiaowei Zhang 1
Oncogene ( IF 6.9 ) Pub Date : 2019-02-28 , DOI: 10.1038/s41388-019-0761-2
Zhengyi Cao 1 , Junhui Xue 1 , Yuning Cheng 1 , Jiyin Wang 1 , Yujuan Liu 1 , Hui Li 1 , Wei Jiang 1 , Gang Li 1 , Yaoting Gui 2 , Xiaowei Zhang 1
Affiliation
|
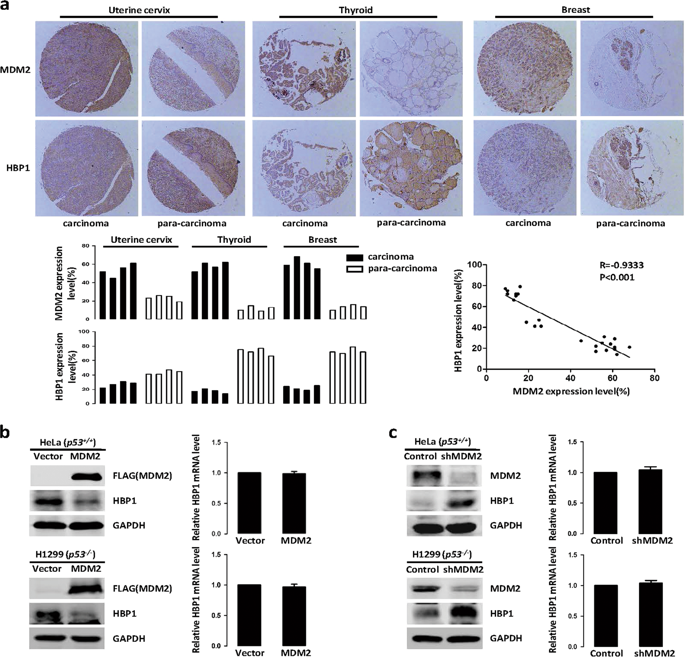
Genome instability is a common feature of tumor cells, and the persistent presence of genome instability is a potential mechanism of tumorigenesis. The E3 ubiquitin ligase MDM2 is intimately involved in genome instability, but its mechanisms are unclear. Our data demonstrated that the transcription factor HBP1 is a target of MDM2. MDM2 facilitates HBP1 proteasomal degradation by ubiquitinating HBP1, regardless of p53 status, thus attenuating the transcriptional inhibition of HBP1 in the expression of its target genes, such as the DNA methyltransferase DNMT1 and histone methyltransferase EZH2, which results in global DNA hypermethylation and histone hypermethylation and ultimately genome instability. The repression of HBP1 by MDM2 finally promotes cell growth and tumorigenesis. Next, we thoroughly explored the regulatory mechanism of the MDM2/HBP1 axis in DNA damage repair following ionizing radiation. Our data indicated that MDM2 overexpression-mediated repression of HBP1 delays DNA damage repair and causes cell death in a p53-independent manner. This investigation elucidated the mechanism of how MDM2 promotes genome instability and enhances tumorigenesis in the absence of p53, thus providing a theoretical and experimental basis for targeting MDM2 as a cancer therapy.
中文翻译:

MDM2通过泛化转录因子HBP1来促进基因组不稳定。
基因组不稳定性是肿瘤细胞的共同特征,而基因组不稳定性的持续存在是肿瘤发生的潜在机制。E3泛素连接酶MDM2与基因组不稳定性密切相关,但其机制尚不清楚。我们的数据表明,转录因子HBP1是MDM2的靶标。MDM2通过泛素化HBP1来促进HBP1蛋白酶体降解,而不考虑p53的状态,从而减弱了HBP1在其靶基因(例如DNA甲基转移酶DNMT1和组蛋白甲基转移酶EZH2)表达中的转录抑制作用,从而导致整体DNA超甲基化和组蛋白超甲基化,以及最终导致基因组不稳定。MDM2抑制HBP1最终促进细胞生长和肿瘤发生。下一个,我们彻底探索了电离辐射后DNA损伤修复中MDM2 / HBP1轴的调控机制。我们的数据表明,MDM2过表达介导的HBP1阻滞延迟DNA损伤修复并以p53独立的方式引起细胞死亡。这项研究阐明了MDM2如何在不存在p53的情况下促进基因组不稳定性并增强肿瘤发生的机制,从而为靶向MDM2作为癌症治疗提供了理论和实验基础。
更新日期:2019-02-28
中文翻译:

MDM2通过泛化转录因子HBP1来促进基因组不稳定。
基因组不稳定性是肿瘤细胞的共同特征,而基因组不稳定性的持续存在是肿瘤发生的潜在机制。E3泛素连接酶MDM2与基因组不稳定性密切相关,但其机制尚不清楚。我们的数据表明,转录因子HBP1是MDM2的靶标。MDM2通过泛素化HBP1来促进HBP1蛋白酶体降解,而不考虑p53的状态,从而减弱了HBP1在其靶基因(例如DNA甲基转移酶DNMT1和组蛋白甲基转移酶EZH2)表达中的转录抑制作用,从而导致整体DNA超甲基化和组蛋白超甲基化,以及最终导致基因组不稳定。MDM2抑制HBP1最终促进细胞生长和肿瘤发生。下一个,我们彻底探索了电离辐射后DNA损伤修复中MDM2 / HBP1轴的调控机制。我们的数据表明,MDM2过表达介导的HBP1阻滞延迟DNA损伤修复并以p53独立的方式引起细胞死亡。这项研究阐明了MDM2如何在不存在p53的情况下促进基因组不稳定性并增强肿瘤发生的机制,从而为靶向MDM2作为癌症治疗提供了理论和实验基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号