当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of 6-Hydroxynicotinate 3-Monooxygenase, a Flavin-Dependent Decarboxylative Hydroxylase Involved in Bacterial Nicotinic Acid Degradation.
Biochemistry ( IF 2.9 ) Pub Date : 2019-03-12 , DOI: 10.1021/acs.biochem.8b00969
Kent D Nakamoto 1 , Scott W Perkins 1 , Ryan G Campbell 1 , Matthew R Bauerle 1 , Tyler J Gerwig 1 , Selim Gerislioglu 2 , Chrys Wesdemiotis 2 , Mark A Anderson 3 , Katherine A Hicks 4 , Mark J Snider 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-03-12 , DOI: 10.1021/acs.biochem.8b00969
Kent D Nakamoto 1 , Scott W Perkins 1 , Ryan G Campbell 1 , Matthew R Bauerle 1 , Tyler J Gerwig 1 , Selim Gerislioglu 2 , Chrys Wesdemiotis 2 , Mark A Anderson 3 , Katherine A Hicks 4 , Mark J Snider 1
Affiliation
|
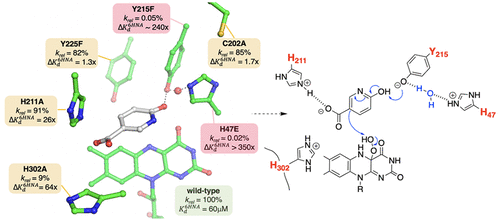
6-Hydroxynicotinate 3-monooxygenase (NicC) is a Group A FAD-dependent monooxygenase that catalyzes the decarboxylative hydroxylation of 6-hydroxynicotinic acid (6-HNA) to 2,5-dihydroxypyridine (2,5-DHP) with concomitant oxidation of NADH in nicotinic acid degradation by aerobic bacteria. Two mechanisms for the decarboxylative hydroxylation half-reaction have been proposed [Hicks, K., et al. (2016) Biochemistry 55, 3432-3446]. Results with Bordetella bronchiseptica RB50 NicC here show that a homocyclic analogue of 6-HNA, 4-hydroxybenzoic acid (4-HBA), is decarboxylated and hydroxylated by NicC with a 420-fold lower catalytic efficiency than is 6-HNA. The 13( V/ K), measured with wild-type NicC by isotope ratio mass spectrometry following the natural abundance of 13C in the CO2 product, is inverse for both 6-HNA (0.9989 ± 0.0002) and 4-HBA (0.9942 ± 0.0004) and becomes negligible (0.9999 ± 0.0004) for 5-chloro-6-HNA, an analogue that is 10-fold more catalytically efficient than 6-HNA. Covalently bound 6-HNA complexes of NicC are not observed by mass spectrometry. Comparative steady-state kinetic and Kd6HNA analyses of active site NicC variants (C202A, H211A, H302A, H47E, Y215F, and Y225F) identify Tyr215 and His47 as critical determinants both of 6-HNA binding ( KdY215F/ KdWT > 240; KdH47E/ KdWT > 350) and in coupling rates of 2,5-DHP and NAD+ product formation ([2,5-DHP]/[NAD+] = 1.00 (WT), 0.005 (Y215F), and 0.07 (H47E)]. Results of these functional analyses are in accord with an electrophilic aromatic substitution reaction mechanism in which His47-Tyr215 may serve as the general base to catalyze substrate hydroxylation and refine the structural model for substrate binding by NicC.
中文翻译:

黄酮依赖性脱羧羟化酶6-羟基烟酸3-单加氧酶参与细菌烟酸降解的机理。
6-羟基烟酸3-单加氧酶(NicC)是A组依赖FAD的单加氧酶,可催化6-羟基烟酸(6-HNA)脱羧化为2,5-二羟基吡啶(2,5-DHP)并伴随NADH的氧化烟酸被需氧细菌降解。已经提出了两种用于脱羧羟基化半反应的机理[Hicks,K。,等人。(2016)Biochemistry 55,3432-3446]。支气管败血性博德特氏菌RB50 NicC的结果表明,6-HNA的同环类似物4-羟基苯甲酸(4-HBA)被NicC脱羧和羟基化,催化效率比6-HNA低420倍。在CO2产物中天然存在13 C之后,通过同位素比率质谱法通过野生型NicC测量的13(V / K)对于6-HNA(0.9989±0.0002)和4-HBA(0。9942±0.0004),而对于5-氯-6-HNA而言,则可以忽略不计(0.9999±0.0004),这是一种比6-HNA催化效率高10倍的类似物。通过质谱未观察到NicC的共价结合的6-HNA复合物。对活性位点NicC变体(C202A,H211A,H302A,H47E,Y215F和Y225F)的稳态动力学和Kd6HNA进行比较分析,确定Tyr215和His47是6-HNA结合的关键决定因素(KdY215F / KdWT> 240; KdH47E / KdWT > 350),以及2,5-DHP和NAD +产物形成的偶联速率([2,5-DHP] / [NAD +] = 1.00(WT),0.005(Y215F)和0.07(H47E)]。
更新日期:2019-02-27
中文翻译:

黄酮依赖性脱羧羟化酶6-羟基烟酸3-单加氧酶参与细菌烟酸降解的机理。
6-羟基烟酸3-单加氧酶(NicC)是A组依赖FAD的单加氧酶,可催化6-羟基烟酸(6-HNA)脱羧化为2,5-二羟基吡啶(2,5-DHP)并伴随NADH的氧化烟酸被需氧细菌降解。已经提出了两种用于脱羧羟基化半反应的机理[Hicks,K。,等人。(2016)Biochemistry 55,3432-3446]。支气管败血性博德特氏菌RB50 NicC的结果表明,6-HNA的同环类似物4-羟基苯甲酸(4-HBA)被NicC脱羧和羟基化,催化效率比6-HNA低420倍。在CO2产物中天然存在13 C之后,通过同位素比率质谱法通过野生型NicC测量的13(V / K)对于6-HNA(0.9989±0.0002)和4-HBA(0。9942±0.0004),而对于5-氯-6-HNA而言,则可以忽略不计(0.9999±0.0004),这是一种比6-HNA催化效率高10倍的类似物。通过质谱未观察到NicC的共价结合的6-HNA复合物。对活性位点NicC变体(C202A,H211A,H302A,H47E,Y215F和Y225F)的稳态动力学和Kd6HNA进行比较分析,确定Tyr215和His47是6-HNA结合的关键决定因素(KdY215F / KdWT> 240; KdH47E / KdWT > 350),以及2,5-DHP和NAD +产物形成的偶联速率([2,5-DHP] / [NAD +] = 1.00(WT),0.005(Y215F)和0.07(H47E)]。








































 京公网安备 11010802027423号
京公网安备 11010802027423号