Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43
Neuron ( IF 14.7 ) Pub Date : 2019-02-27 , DOI: 10.1016/j.neuron.2019.01.048 Jacob R Mann 1 , Amanda M Gleixner 2 , Jocelyn C Mauna 2 , Edward Gomes 3 , Michael R DeChellis-Marks 1 , Patrick G Needham 4 , Katie E Copley 2 , Bryan Hurtle 2 , Bede Portz 3 , Noah J Pyles 2 , Lin Guo 3 , Christopher B Calder 2 , Zachary P Wills 5 , Udai B Pandey 6 , Julia K Kofler 7 , Jeffrey L Brodsky 8 , Amantha Thathiah 5 , James Shorter 3 , Christopher J Donnelly 9
Neuron ( IF 14.7 ) Pub Date : 2019-02-27 , DOI: 10.1016/j.neuron.2019.01.048 Jacob R Mann 1 , Amanda M Gleixner 2 , Jocelyn C Mauna 2 , Edward Gomes 3 , Michael R DeChellis-Marks 1 , Patrick G Needham 4 , Katie E Copley 2 , Bryan Hurtle 2 , Bede Portz 3 , Noah J Pyles 2 , Lin Guo 3 , Christopher B Calder 2 , Zachary P Wills 5 , Udai B Pandey 6 , Julia K Kofler 7 , Jeffrey L Brodsky 8 , Amantha Thathiah 5 , James Shorter 3 , Christopher J Donnelly 9
Affiliation
|
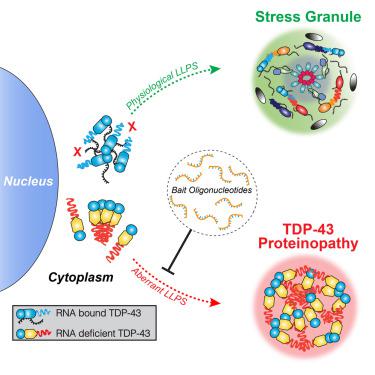
TDP-43 proteinopathy is a pathological hallmark of amyotrophic lateral sclerosis and frontotemporal dementia where cytoplasmic TDP-43 inclusions are observed within degenerating regions of patient postmortem tissue. The mechanism by which TDP-43 aggregates has remained elusive due to technological limitations, which prevent the analysis of specific TDP-43 interactions in live cells. We present an optogenetic approach to reliably induce TDP-43 proteinopathy under spatiotemporal control. We show that the formation of pathologically relevant inclusions is driven by aberrant interactions between low-complexity domains of TDP-43 that are antagonized by RNA binding. Although stress granules are hypothesized to be a conduit for seeding TDP-43 proteinopathy, we demonstrate pathological inclusions outside these RNA-rich structures. Furthermore, we show that aberrant phase transitions of cytoplasmic TDP-43 are neurotoxic and that treatment with oligonucleotides composed of TDP-43 target sequences prevent inclusions and rescue neurotoxicity. Collectively, these studies provide insight into the mechanisms that underlie TDP-43 proteinopathy and present a potential avenue for therapeutic intervention.
中文翻译:

RNA 结合拮抗 TDP-43 的神经毒性相变
TDP-43 蛋白病是肌萎缩侧索硬化症和额颞叶痴呆的病理标志,其中在患者死后组织的退化区域内观察到细胞质 TDP-43 包涵体。由于技术限制,TDP-43 聚集的机制仍然难以捉摸,这阻止了对活细胞中特定 TDP-43 相互作用的分析。我们提出了一种在时空控制下可靠诱导 TDP-43 蛋白病的光遗传学方法。我们表明,病理相关包涵体的形成是由 TDP-43 的低复杂性结构域之间的异常相互作用驱动的,这些结构域被 RNA 结合拮抗。尽管假设应激颗粒是接种 TDP-43 蛋白病的管道,但我们证明了这些富含 RNA 的结构之外的病理包涵体。此外,我们表明细胞质 TDP-43 的异常相变具有神经毒性,并且用由 TDP-43 靶序列组成的寡核苷酸处理可以防止包涵体并挽救神经毒性。总的来说,这些研究提供了对 TDP-43 蛋白病基础机制的见解,并为治疗干预提供了一条潜在的途径。
更新日期:2019-02-27
中文翻译:

RNA 结合拮抗 TDP-43 的神经毒性相变
TDP-43 蛋白病是肌萎缩侧索硬化症和额颞叶痴呆的病理标志,其中在患者死后组织的退化区域内观察到细胞质 TDP-43 包涵体。由于技术限制,TDP-43 聚集的机制仍然难以捉摸,这阻止了对活细胞中特定 TDP-43 相互作用的分析。我们提出了一种在时空控制下可靠诱导 TDP-43 蛋白病的光遗传学方法。我们表明,病理相关包涵体的形成是由 TDP-43 的低复杂性结构域之间的异常相互作用驱动的,这些结构域被 RNA 结合拮抗。尽管假设应激颗粒是接种 TDP-43 蛋白病的管道,但我们证明了这些富含 RNA 的结构之外的病理包涵体。此外,我们表明细胞质 TDP-43 的异常相变具有神经毒性,并且用由 TDP-43 靶序列组成的寡核苷酸处理可以防止包涵体并挽救神经毒性。总的来说,这些研究提供了对 TDP-43 蛋白病基础机制的见解,并为治疗干预提供了一条潜在的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号