当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Bioactivity of Quinoline‐3‐carboxamide Derivatives
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2018-02-08 , DOI: 10.1002/jhet.3132 Hogantharanni Govender 1 , Chunderika Mocktar 1 , Neil A. Koorbanally 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2018-02-08 , DOI: 10.1002/jhet.3132 Hogantharanni Govender 1 , Chunderika Mocktar 1 , Neil A. Koorbanally 1
Affiliation
|
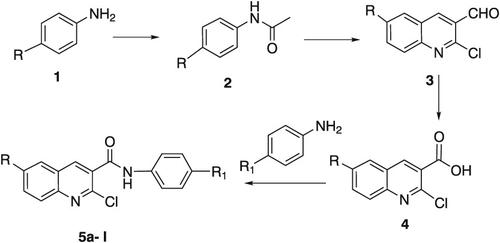
Twelve novel substituted 2‐chloroquinoline‐3‐carboxamide derivatives were prepared from acetanilides using the Vilsmeier–Haack reaction, producing 2‐chloro‐3‐carbaldehyde quinolines, followed by oxidation of the 3‐carbaldehyde to the carboxylic acid and coupling this group with various anilines. The structures of the synthesized compounds were confirmed by NMR, mass spectrometry, and single crystal X‐ray diffraction. The chemical shifts of H‐5 and H‐8 were shown to be influenced by the substituent at C‐6. The substituent at C‐6 was also seen to affect the chemical shift of C‐5, C‐7, and C‐8, with C‐5 and C‐7 being more shielded in 5j (F substituted) in comparison with 5g (Cl substituted) and 5d (CH3 substituted). The compounds showed weak activity in the mM range against Gram‐positive and Gram‐negative bacteria of which 5b, 5d, and 5f showed the best activity with minimum bactericidal concentration values for 5b being 3.79 mM against methicillin‐resistant Staphylococcus aureus and 5d and 5f having minimum bactericidal concentration values of 3.77 and 1.79 mM against S. aureus ATCC 25923, respectively.
中文翻译:

喹啉-3-羧酰胺衍生物的合成及生物活性
使用Vilsmeier-Haack反应,由乙苯胺制备十二种新颖的2-氯喹啉-3-羧酰胺衍生物,生成2-氯-3-羰基甲醛喹啉,然后将3-甲醛氧化成羧酸,并将该基团与各种苯胺。合成的化合物的结构已通过NMR,质谱和单晶X射线衍射确认。H-5和H-8的化学位移显示受C-6取代基的影响。在C-6上的取代基也被视为影响C-5,C-7和C-8,的化学位移与C-5和C-7中被更屏蔽5J(F取代的)在比较5克(取代的Cl)和5d(CH 3替代)。这些化合物在mM范围内对革兰氏阳性和革兰氏阴性细菌的活性较弱,其中5b,5d和5f表现出最佳的活性,而对5b的最小杀菌浓度值为3.79 mM,对耐甲氧西林的金黄色葡萄球菌以及5d和5f对金黄色葡萄球菌ATCC 25923的最小杀菌浓度分别为3.77和1.79 mM 。
更新日期:2018-02-08
中文翻译:

喹啉-3-羧酰胺衍生物的合成及生物活性
使用Vilsmeier-Haack反应,由乙苯胺制备十二种新颖的2-氯喹啉-3-羧酰胺衍生物,生成2-氯-3-羰基甲醛喹啉,然后将3-甲醛氧化成羧酸,并将该基团与各种苯胺。合成的化合物的结构已通过NMR,质谱和单晶X射线衍射确认。H-5和H-8的化学位移显示受C-6取代基的影响。在C-6上的取代基也被视为影响C-5,C-7和C-8,的化学位移与C-5和C-7中被更屏蔽5J(F取代的)在比较5克(取代的Cl)和5d(CH 3替代)。这些化合物在mM范围内对革兰氏阳性和革兰氏阴性细菌的活性较弱,其中5b,5d和5f表现出最佳的活性,而对5b的最小杀菌浓度值为3.79 mM,对耐甲氧西林的金黄色葡萄球菌以及5d和5f对金黄色葡萄球菌ATCC 25923的最小杀菌浓度分别为3.77和1.79 mM 。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号