当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering of Cyclohexanone Monooxygenase for the Enantioselective Synthesis of (S)-Omeprazole
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-02-27 00:00:00 , DOI: 10.1021/acssuschemeng.9b00224 Yan Zhang 1 , Yin-Qi Wu 1 , Na Xu 1 , Qian Zhao 2 , Hui-Lei Yu 1 , Jian-He Xu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-02-27 00:00:00 , DOI: 10.1021/acssuschemeng.9b00224 Yan Zhang 1 , Yin-Qi Wu 1 , Na Xu 1 , Qian Zhao 2 , Hui-Lei Yu 1 , Jian-He Xu 1
Affiliation
|
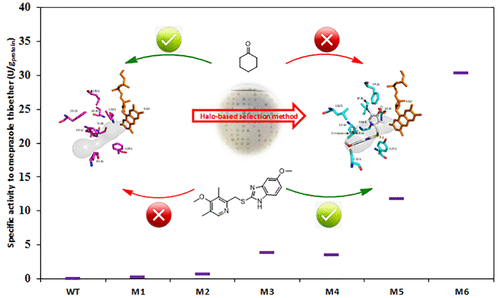
Enzymatic asymmetric sulfoxidation using molecular oxygen as the oxidant is a promising green chemistry approach to chiral sulfoxide production. Despite the broad substrate spectrum of cyclohexanone monooxygenases (CHMOs), some unnatural substrates with bulky functional groups, such as the pharmaceutically relevant omeprazole sulfide, cannot be effectively accepted by CHMOs. Herein, we describe a set of variants derived from an Acinetobacter calcoaceticus CHMO (AcCHMO), whose active sites adjacent to the substrate tunnel were altered to shift the substrate specificity from cyclohexanone monooxygenation toward omeprazole sulfide sulfoxidation. We performed homologous modeling and molecular docking to identify key residues that might affect the substrate specificity. Two libraries of residues lining the active center of AcCHMO were then constructed and screened by an effective halo-based selection method using the solubility difference between the substrate (omeprazole sulfide) and product (esomeprazole). Functional evaluation of the resultant variants showed that the substrate specificity of AcCHMO was markedly altered from the small natural substrate (cyclohexanone) toward the desired bulky substrate (omeprazole sulfide) despite the extremely poor activity detected even for the best variant, M2 (0.61 U/gprot). The crystal structure of M2 complexed with a flavin adenine dinucleotide (FAD) prosthetic group was determined, which provided insight into the altered substrate specificity. To improve the activity of enzyme M2 toward pharmaceutical precursor omeprazole sulfide, we performed both local and global protein engineering among the two CASTing libraries surrounding FAD+ and NADP+ prosthetic groups and an error-prone PCR library of the full-length AcCHMO. As a result, variant M6 was obtained, giving a 50-fold higher activity compared to M2. This structure-guided protein engineering of AcCHMO provided a promising candidate for converting omeprazole sulfide into (S)-omeprazole using a green biocatalytic method.
中文翻译:

对映体选择性合成(S)-奥美拉唑的环己酮单加氧酶工程
使用分子氧作为氧化剂的酶促不对称硫氧化是一种有前途的绿色化学方法,用于手性亚砜的生产。尽管环己酮单加氧酶(CHMO)的底物谱范围很广,但是一些具有庞大功能基团的非天然底物(例如与药物相关的奥美拉唑硫化物)不能被CHMO有效地接受。在本文中,我们描述了一组源自钙不动杆菌不动杆菌CHMO(AcCHMO),其活性位点与底物通道相邻处发生了改变,以将底物特异性从环己酮单加氧转变为奥美拉唑硫化物硫氧化。我们进行了同源建模和分子对接,以鉴定可能影响底物特异性的关键残基。然后,使用底物(奥美拉唑硫化物)和产物(埃索美拉唑)之间的溶解度差异,通过有效的基于卤素的选择方法构建并筛选了两个Ac AcMO活性中心衬里的残基文库。所得变体的功能评估表明Ac的底物特异性尽管已检测到即使对于最佳变体M2(0.61 U / g prot)而言,活性也极差,但CHMO仍从小的天然底物(环己酮)向所需的大体积底物(奥美拉唑硫化物)显着改变。确定了与黄素腺嘌呤二核苷酸(FAD)修复基团复合的M2的晶体结构,这为改变底物特异性提供了见识。为了提高酶M2对药物前体奥美拉唑硫化物的活性,我们在围绕FAD +和NADP +修复基团的两个CASTing文库和全长Ac的容易出错的PCR文库中进行了局部和全局蛋白工程设计CHMO。结果,获得变体M6,其活性是M2的50倍。Ac CHMO的这种结构指导的蛋白质工程为使用绿色生物催化方法将奥美拉唑硫化物转化为(S)-奥美拉唑提供了有希望的候选者。
更新日期:2019-02-27
中文翻译:

对映体选择性合成(S)-奥美拉唑的环己酮单加氧酶工程
使用分子氧作为氧化剂的酶促不对称硫氧化是一种有前途的绿色化学方法,用于手性亚砜的生产。尽管环己酮单加氧酶(CHMO)的底物谱范围很广,但是一些具有庞大功能基团的非天然底物(例如与药物相关的奥美拉唑硫化物)不能被CHMO有效地接受。在本文中,我们描述了一组源自钙不动杆菌不动杆菌CHMO(AcCHMO),其活性位点与底物通道相邻处发生了改变,以将底物特异性从环己酮单加氧转变为奥美拉唑硫化物硫氧化。我们进行了同源建模和分子对接,以鉴定可能影响底物特异性的关键残基。然后,使用底物(奥美拉唑硫化物)和产物(埃索美拉唑)之间的溶解度差异,通过有效的基于卤素的选择方法构建并筛选了两个Ac AcMO活性中心衬里的残基文库。所得变体的功能评估表明Ac的底物特异性尽管已检测到即使对于最佳变体M2(0.61 U / g prot)而言,活性也极差,但CHMO仍从小的天然底物(环己酮)向所需的大体积底物(奥美拉唑硫化物)显着改变。确定了与黄素腺嘌呤二核苷酸(FAD)修复基团复合的M2的晶体结构,这为改变底物特异性提供了见识。为了提高酶M2对药物前体奥美拉唑硫化物的活性,我们在围绕FAD +和NADP +修复基团的两个CASTing文库和全长Ac的容易出错的PCR文库中进行了局部和全局蛋白工程设计CHMO。结果,获得变体M6,其活性是M2的50倍。Ac CHMO的这种结构指导的蛋白质工程为使用绿色生物催化方法将奥美拉唑硫化物转化为(S)-奥美拉唑提供了有希望的候选者。

































 京公网安备 11010802027423号
京公网安备 11010802027423号