当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of Spiro-2-oxabicyclo[2.2.2]octane Enabled by Ag(I)/ Brønsted Acid Relay Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2019-02-26 00:00:00 , DOI: 10.1021/acs.orglett.9b00251 Qingyu Zhang 1 , Jianping Wang 1 , Yansheng Wei 1 , Hongbin Zhai 2 , Yun Li 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2019-02-26 00:00:00 , DOI: 10.1021/acs.orglett.9b00251 Qingyu Zhang 1 , Jianping Wang 1 , Yansheng Wei 1 , Hongbin Zhai 2 , Yun Li 1, 3
Affiliation
|
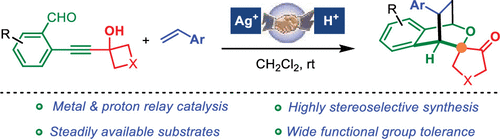
A highly stereoselective synthesis of a spiro-cineole scaffold that contains four stereogenic centers from readily accessible 2-alkynylbenzaldehydes and styrenes under very mild reaction conditions was reported. This cascade reaction involves a Ag(I)-catalyzed alkyne cycloisomerization and oxa-[4 + 2]-cycloaddition to give an oxonium intermediate which subsequently undergoes a previously unexplored 1,2-alkyl migration to access highly strained spiro-2-oxabicyclo[2.2.2]octanes in high yields with excellent stereoselectivities.
中文翻译:

Ag(I)/Brønsted酸继电催化实现螺-2-氧杂双环[2.2.2]辛烷的立体选择性合成
报道了在非常温和的反应条件下,由容易获得的2-炔基苯甲醛和苯乙烯包含四个立体异构中心的螺-桉树脑骨架的高度立体选择性合成。该级联反应涉及一种由Ag(I)催化的炔烃环异构化和oxa- [4 + 2]-环加成反应生成的氧鎓中间体,该中间体随后经历了先前未经探索的1,2-烷基迁移,从而获得了高度应变的spiro-2-oxabicyclocyclo [ 2.2.2]高产率的辛烷,具有出色的立体选择性。
更新日期:2019-02-26
中文翻译:

Ag(I)/Brønsted酸继电催化实现螺-2-氧杂双环[2.2.2]辛烷的立体选择性合成
报道了在非常温和的反应条件下,由容易获得的2-炔基苯甲醛和苯乙烯包含四个立体异构中心的螺-桉树脑骨架的高度立体选择性合成。该级联反应涉及一种由Ag(I)催化的炔烃环异构化和oxa- [4 + 2]-环加成反应生成的氧鎓中间体,该中间体随后经历了先前未经探索的1,2-烷基迁移,从而获得了高度应变的spiro-2-oxabicyclocyclo [ 2.2.2]高产率的辛烷,具有出色的立体选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号