当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Morphology and Magnetic Structure of the Ferritin Core during Iron Loading and Release by Magnetooptical and NMR Methods
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsami.7b18304 Marceli Koralewski 1 , Lucia Balejčíková 2, 3 , Zuzana Mitróová 2 , Mikołaj Pochylski 1 , Mikołaj Baranowski 1 , Peter Kopčanský 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acsami.7b18304 Marceli Koralewski 1 , Lucia Balejčíková 2, 3 , Zuzana Mitróová 2 , Mikołaj Pochylski 1 , Mikołaj Baranowski 1 , Peter Kopčanský 2
Affiliation
|
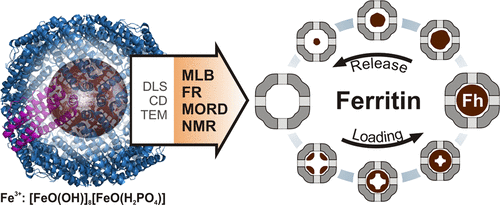
Ferritins are proteins, which serve as a storage and transportation capsule for iron inside living organisms. Continuously charging the proteins with iron and releasing it from the ferritin is necessary to assure proper management of these important ions within the organism. On the other hand, synthetic ferritins have great potential for biomedical and technological applications. In this work, the behavior of ferritin during the processes of iron loading and release was examined using multiplicity of the experimental technique. The quality of the protein’s shell was monitored using circular dichroism, whereas the average size and its distribution were estimated from dynamic light scattering and transmission electron microscopy images, respectively. Because of the magnetic behavior of the iron mineral, a number of magnetooptical methods were used to gain information on the iron core of the ferritin. Faraday rotation and magnetic linear birefringence studies provide evidence that the iron loading and the iron-release processes are not symmetrical. The spatial organization of the mineral within the protein’s core changes depending on whether the iron was incorporated into or removed from the ferritin’s shell. Magnetic optical rotatory dispersion spectra exclude the contribution of the Fe(II)-composed mineral, whereas joined magnetooptical and nuclear magnetic resonance results indicate that no mineral with high magnetization appear at any stage of the loading/release process. These findings suggest that the iron core of loaded/released ferritin consists of single-phase, that is, ferrihydrite. The presented results demonstrate the usefulness of emerging magnetooptical methods in biomedical research and applications.
中文翻译:

磁光法和核磁共振法测定铁负载和释放过程中铁蛋白核的形貌和磁性结构
铁蛋白是蛋白质,可作为生物体内铁的存储和运输囊。为了确保对生物体内这些重要离子的适当管理,有必要对铁连续充电并使蛋白质从铁蛋白中释放出来。另一方面,合成铁蛋白在生物医学和技术应用方面具有巨大潜力。在这项工作中,使用多种实验技术检查了铁蛋白在铁负载和释放过程中的行为。蛋白质的外壳质量使用圆二色性进行监控,而平均大小及其分布则分别通过动态光散射和透射电子显微镜图像进行估算。由于铁矿物质的磁性,许多磁光方法用于获得有关铁蛋白铁心的信息。法拉第旋转和磁性线性双折射研究提供了铁负载和铁释放过程不对称的证据。蛋白质核心中矿物质的空间组织会根据铁是结合到铁蛋白的壳中还是从铁蛋白的壳中除去而发生变化。磁性旋光色散谱排除了由Fe(II)组成的矿物的贡献,而结合的磁光和核磁共振结果表明在加载/释放过程的任何阶段都没有出现具有高磁化强度的矿物。这些发现表明,加载/释放的铁蛋白的铁芯由单相组成,即水铁矿。
更新日期:2018-02-08
中文翻译:

磁光法和核磁共振法测定铁负载和释放过程中铁蛋白核的形貌和磁性结构
铁蛋白是蛋白质,可作为生物体内铁的存储和运输囊。为了确保对生物体内这些重要离子的适当管理,有必要对铁连续充电并使蛋白质从铁蛋白中释放出来。另一方面,合成铁蛋白在生物医学和技术应用方面具有巨大潜力。在这项工作中,使用多种实验技术检查了铁蛋白在铁负载和释放过程中的行为。蛋白质的外壳质量使用圆二色性进行监控,而平均大小及其分布则分别通过动态光散射和透射电子显微镜图像进行估算。由于铁矿物质的磁性,许多磁光方法用于获得有关铁蛋白铁心的信息。法拉第旋转和磁性线性双折射研究提供了铁负载和铁释放过程不对称的证据。蛋白质核心中矿物质的空间组织会根据铁是结合到铁蛋白的壳中还是从铁蛋白的壳中除去而发生变化。磁性旋光色散谱排除了由Fe(II)组成的矿物的贡献,而结合的磁光和核磁共振结果表明在加载/释放过程的任何阶段都没有出现具有高磁化强度的矿物。这些发现表明,加载/释放的铁蛋白的铁芯由单相组成,即水铁矿。

































 京公网安备 11010802027423号
京公网安备 11010802027423号