当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodosobenzene-Mediated α-Acyloxylation of 1,3-Dicarbonyl Compounds with Carboxylic Acids and Insight into the Reaction Mechanism
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.joc.8b00114 Chitturi Bhujanga Rao 1 , Jingwen Yuan 1 , Qian Zhang 1 , Rui Zhang 1 , Ning Zhang 1, 2 , Jianyong Fang 1, 2 , Dewen Dong 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.joc.8b00114 Chitturi Bhujanga Rao 1 , Jingwen Yuan 1 , Qian Zhang 1 , Rui Zhang 1 , Ning Zhang 1, 2 , Jianyong Fang 1, 2 , Dewen Dong 1, 2
Affiliation
|
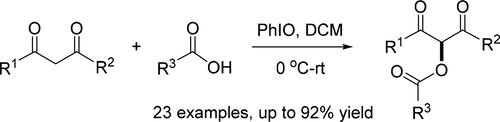
A highly efficient direct α-acyloxylation of 1,3-dicarbonyl compounds with carboxylic acids mediated by hypervalent iodine reagent is presented. Treatment of a variety of 1,3-dicarbonyl compounds with carboxylic acids in the presence of iodosobenzene provides the corresponding α-acyloxylated products in good to excellent yields. The mechanistic investigation by means of NMR spectroscopy reveals that the in situ-generated phenyliodine biscarboxylate proves to be the key intermediate for the α-acyloxylation, and the loading sequence of reactants and oxidant is crucial for the generation of the active species. The mild reaction conditions, wide substrate scope, short reaction time, good yields, high chemoselectivity, excellent functional group tolerance, and metal catalyst-free conversion make this acyloxylation a significant synthetic protocol.
中文翻译:

碘代苯介导的1,3-二羰基化合物与羧酸的α-酰氧基化反应及机理研究
提出了一种由高价碘试剂介导的高效1,3-二羰基化合物与羧酸的直接α-酰氧基化反应。在碘代苯的存在下用羧酸处理各种1,3-二羰基化合物可提供相应的α-酰氧基化产物,收率良好至极佳。通过NMR光谱的机理研究表明,原位生成的苯基碘双羧酸盐被证明是α-酰氧基化的关键中间体,并且反应物和氧化剂的装载顺序对于活性物质的产生是至关重要的。温和的反应条件,宽泛的底物范围,短的反应时间,良好的收率,高的化学选择性,出色的官能团耐受性和无金属催化剂的转化,使得该酰氧基化成为重要的合成方案。
更新日期:2018-02-19
中文翻译:

碘代苯介导的1,3-二羰基化合物与羧酸的α-酰氧基化反应及机理研究
提出了一种由高价碘试剂介导的高效1,3-二羰基化合物与羧酸的直接α-酰氧基化反应。在碘代苯的存在下用羧酸处理各种1,3-二羰基化合物可提供相应的α-酰氧基化产物,收率良好至极佳。通过NMR光谱的机理研究表明,原位生成的苯基碘双羧酸盐被证明是α-酰氧基化的关键中间体,并且反应物和氧化剂的装载顺序对于活性物质的产生是至关重要的。温和的反应条件,宽泛的底物范围,短的反应时间,良好的收率,高的化学选择性,出色的官能团耐受性和无金属催化剂的转化,使得该酰氧基化成为重要的合成方案。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号