当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Electrocatalytic Activity of Ammonium Nickel Phosphate, [NH4]NiPO4·6H2O, and β-Nickel Pyrophosphate, β-Ni2P2O7: Catalysts for Electrocatalytic Decomposition of Urea
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02658
Andrew G. Meguerdichian 1 , Tahereh Jafari 1 , Md. R. Shakil 2 , Ran Miao 2 , Laura A. Achola 2 , John Macharia 2 , Alireza Shirazi-Amin 2 , Steven L. Suib 1, 2, 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02658
Andrew G. Meguerdichian 1 , Tahereh Jafari 1 , Md. R. Shakil 2 , Ran Miao 2 , Laura A. Achola 2 , John Macharia 2 , Alireza Shirazi-Amin 2 , Steven L. Suib 1, 2, 3
Affiliation
|
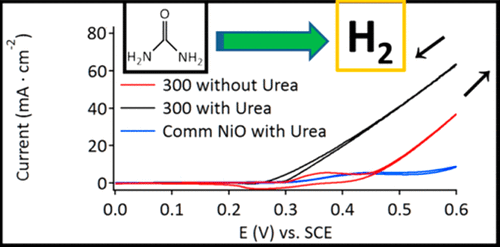
Electrocatalytic decomposition of urea for the production of hydrogen, H2, for clean energy applications, such as in fuel cells, has several potential advantages such as reducing carbon emissions in the energy sector and environmental applications to remove urea from animal and human waste facilities. The study and development of new catalyst materials containing nickel metal, the active site for urea decomposition, is a critical aspect of research in inorganic and materials chemistry. We report the synthesis and application of [NH4]NiPO4·6H2O and β-Ni2P2O7 using in situ prepared [NH4]2HPO4. The [NH4]NiPO4·6H2O is calcined at varying temperatures and tested for electrocatalytic decomposition of urea. Our results indicate that [NH4]NiPO4·6H2O calcined at 300 °C with an amorphous crystal structure and, for the first time applied for urea electrocatalytic decomposition, had the greatest reported electroactive surface area (ESA) of 142 cm2/mg and an onset potential of 0.33 V (SCE) and was stable over a 24-h test period.
中文翻译:

铵磷酸镍的合成及电化学性质,[NH 4 ] NIPO 4 ·6H 2 O,和β-焦磷酸镍,β-Ni系2 P 2 ö 7:催化剂为尿素的电催化分解
用于清洁能源应用(例如燃料电池)的尿素的电催化分解产生氢气H 2具有数个潜在的优势,例如减少能源部门的碳排放量以及在环境应用中从动物和人类废物设施中去除尿素。含镍金属(尿素分解的活性部位)的新型催化剂材料的研究和开发是无机化学和材料化学研究的重要方面。我们报告的[NH合成和应用4 ] NIPO 4 ·6H 2 O和的β-Ni 2 P 2 ö 7使用原位制备的[NH 4 ] 2HPO 4。[NH 4 ] NiPO 4 ·6H 2 O在不同的温度下煅烧并测试尿素的电催化分解。我们的结果表明,[NH 4 ] NiPO 4 ·6H 2 O在300°C下煅烧,具有无定形晶体结构,并且首次用于尿素电催化分解,具有最大的电活性表面积(ESA)为142 cm 2。 / mg,起始电位为0.33 V(SCE),在24小时的测试期间内保持稳定。
更新日期:2018-02-08
中文翻译:

铵磷酸镍的合成及电化学性质,[NH 4 ] NIPO 4 ·6H 2 O,和β-焦磷酸镍,β-Ni系2 P 2 ö 7:催化剂为尿素的电催化分解
用于清洁能源应用(例如燃料电池)的尿素的电催化分解产生氢气H 2具有数个潜在的优势,例如减少能源部门的碳排放量以及在环境应用中从动物和人类废物设施中去除尿素。含镍金属(尿素分解的活性部位)的新型催化剂材料的研究和开发是无机化学和材料化学研究的重要方面。我们报告的[NH合成和应用4 ] NIPO 4 ·6H 2 O和的β-Ni 2 P 2 ö 7使用原位制备的[NH 4 ] 2HPO 4。[NH 4 ] NiPO 4 ·6H 2 O在不同的温度下煅烧并测试尿素的电催化分解。我们的结果表明,[NH 4 ] NiPO 4 ·6H 2 O在300°C下煅烧,具有无定形晶体结构,并且首次用于尿素电催化分解,具有最大的电活性表面积(ESA)为142 cm 2。 / mg,起始电位为0.33 V(SCE),在24小时的测试期间内保持稳定。

































 京公网安备 11010802027423号
京公网安备 11010802027423号