当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inner-Sphere and Outer-Sphere Water Interactions in Co(II) paraCEST Agents
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02977 Samira M. Abozeid 1 , Eric M. Snyder 1 , Timothy Y. Tittiris 1 , Charles M. Steuerwald 1 , Alexander Y. Nazarenko 2 , Janet R. Morrow 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02977 Samira M. Abozeid 1 , Eric M. Snyder 1 , Timothy Y. Tittiris 1 , Charles M. Steuerwald 1 , Alexander Y. Nazarenko 2 , Janet R. Morrow 1
Affiliation
|
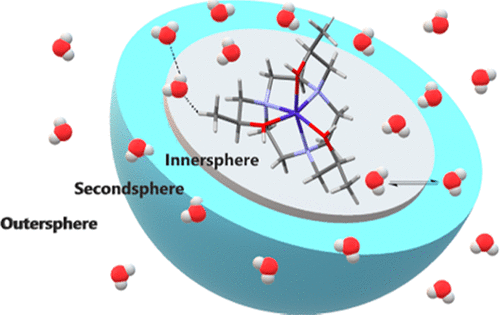
High-spin Co(II) complexes are promising for development as paraCEST agents (paraCEST = paramagnetic chemical exchange saturation transfer) for magnetic resonance imaging applications. The first examples of Co(II) paraCEST agents with bound water ligands are presented here. Four Co(II) macrocyclic complexes based on 1,4,7-triazacyclononane and containing either pendent alcohol or pendent amide groups were prepared. Two of the macrocycles encapsulate the Co(II) and contain no water ligands as shown by X-ray crystallographic studies, and two complexes have macrocycles with only five ligand donor groups to leave an open coordination site for bound water. The ionization of alcohol, water, or amide groups in the complexes was characterized by using pH potentiometry. These data show that one of the complexes has a readily deprotonated group with a pKa close to 6, which is assigned as an alcohol pendent. Amide pendents deprotonate at high pH (>8), and the water ligands of the Co(II) complexes are not deprotonated at neutral pH. All complexes produce CEST peaks through either alcohol OH or amide NH proton exchange. The water ligands exchange too rapidly to produce a CEST effect as shown by variable-temperature 17O NMR spectroscopy studies. The complexes with available coordination sites for inner-sphere water ligands produce large paramagnetic shifts and broadening of the 17O resonances of bulk water, whereas the encapsulated complexes show much less shifting and broadening of 17O resonances. All complexes produce substantial paramagnetic shifts of the 1H resonances of bulk water, which is promising for the development of supramolecular CEST agents.
中文翻译:

Co(II)paraCEST剂中的内层和外层水相互作用
高自旋Co(II)配合物有望作为磁共振成像应用的paraCEST试剂(paraCEST =顺磁性化学交换饱和转移)发展。本文介绍了具有结合水配体的Co(II)paraCEST试剂的第一个实例。制备了四个基于1,4,7-三氮杂环壬烷并含有侧链醇或侧链酰胺基的Co(II)大环配合物。X射线晶体学研究显示,其中两个大环封装了Co(II),不包含水配体,两个配合物的大环中只有五个配体供体基团,为结合的水留下了开放的配位点。配合物中醇,水或酰胺基团的电离通过pH电位法进行表征。这些数据表明,其中一种配合物具有易于去质子化的ap基团。K a接近于6,它被指定为酒精下垂剂。酰胺悬垂剂在高pH(> 8)下会去质子化,而Co(II)配合物的水配体在中性pH下不会去质子化。所有复合物都会通过醇OH或酰胺NH质子交换产生CEST峰。如变温17 O NMR光谱研究所示,水配体交换太快而无法产生CEST效果。具有可用于内球水配体的配位点的配合物会产生大的顺磁位移,并使散装水的17 O共振变宽,而封装的配合物则显示出更少的17 O共振位移和变宽。所有配合物产生的显着的顺磁位移1大量水的H共振,对于超分子CEST试剂的开发是有希望的。
更新日期:2018-02-07
中文翻译:

Co(II)paraCEST剂中的内层和外层水相互作用
高自旋Co(II)配合物有望作为磁共振成像应用的paraCEST试剂(paraCEST =顺磁性化学交换饱和转移)发展。本文介绍了具有结合水配体的Co(II)paraCEST试剂的第一个实例。制备了四个基于1,4,7-三氮杂环壬烷并含有侧链醇或侧链酰胺基的Co(II)大环配合物。X射线晶体学研究显示,其中两个大环封装了Co(II),不包含水配体,两个配合物的大环中只有五个配体供体基团,为结合的水留下了开放的配位点。配合物中醇,水或酰胺基团的电离通过pH电位法进行表征。这些数据表明,其中一种配合物具有易于去质子化的ap基团。K a接近于6,它被指定为酒精下垂剂。酰胺悬垂剂在高pH(> 8)下会去质子化,而Co(II)配合物的水配体在中性pH下不会去质子化。所有复合物都会通过醇OH或酰胺NH质子交换产生CEST峰。如变温17 O NMR光谱研究所示,水配体交换太快而无法产生CEST效果。具有可用于内球水配体的配位点的配合物会产生大的顺磁位移,并使散装水的17 O共振变宽,而封装的配合物则显示出更少的17 O共振位移和变宽。所有配合物产生的显着的顺磁位移1大量水的H共振,对于超分子CEST试剂的开发是有希望的。































 京公网安备 11010802027423号
京公网安备 11010802027423号