当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Ecumicin
Organic Letters ( IF 4.9 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03967 Paige M. E. Hawkins 1 , Andrew M. Giltrap 1 , Gayathri Nagalingam 2 , Warwick J. Britton 2 , Richard J. Payne 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03967 Paige M. E. Hawkins 1 , Andrew M. Giltrap 1 , Gayathri Nagalingam 2 , Warwick J. Britton 2 , Richard J. Payne 1
Affiliation
|
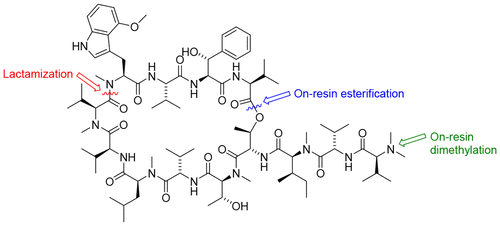
The first total synthesis of the potent anti-mycobacterial cyclic depsipeptide natural product ecumicin is described. Synthesis was achieved via a solid-phase strategy, incorporating the synthetic non-proteinogenic amino acids N-methyl-4-methoxy-l-tryptophan and threo-β-hydroxy-l-phenylalanine into the growing linear peptide chain. The synthesis employed key on-resin esterification and dimethylation steps as well as a final macrolactamization between the unusual N-methyl-4-methoxy-l-tryptophan unit and a bulky N-methyl-l-valine residue. The synthetic natural product possessed potent antimycobacterial activity against the virulent H37Rv strain of Mycobacterium tuberculosis (MIC90 = 312 nM).
中文翻译:

紫cum素的全合成
描述了有效的抗分枝杆菌环状二肽天然产物天然紫杉醇的第一全合成。通过固相策略实现合成,将合成的非蛋白质氨基酸N-甲基-4-甲氧基-1-色氨酸和苏氨酸-β-羟基-1-苯丙氨酸掺入正在增长的线性肽链中。合成中使用的关键在树脂上酯化和二甲基化的步骤以及异常之间的最终macrolactamization Ñ甲基-4-甲氧基-升-色氨酸单元和笨重Ñ甲基升-缬氨酸残基。合成的天然产物对结核分枝杆菌的强毒H37Rv菌株(MIC 90 = 312 nM)具有有效的抗分枝杆菌活性。
更新日期:2018-02-07
中文翻译:

紫cum素的全合成
描述了有效的抗分枝杆菌环状二肽天然产物天然紫杉醇的第一全合成。通过固相策略实现合成,将合成的非蛋白质氨基酸N-甲基-4-甲氧基-1-色氨酸和苏氨酸-β-羟基-1-苯丙氨酸掺入正在增长的线性肽链中。合成中使用的关键在树脂上酯化和二甲基化的步骤以及异常之间的最终macrolactamization Ñ甲基-4-甲氧基-升-色氨酸单元和笨重Ñ甲基升-缬氨酸残基。合成的天然产物对结核分枝杆菌的强毒H37Rv菌株(MIC 90 = 312 nM)具有有效的抗分枝杆菌活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号