Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-02-25 , DOI: 10.1016/j.bioorg.2019.02.051 Fariba Peytam 1 , Mehdi Adib 1 , Shabnam Mahernia 2 , Mahmoud Rahmanian-Jazi 1 , Mehdi Jahani 1 , Behrad Masoudi 1 , Mohammad Mahdavi 3 , Massoud Amanlou 4
|
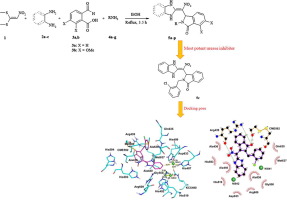
An efficient, one-pot and four-component synthesis of a new series of 2,3-disubstituted isoindolin-1-ones is described and their Jack bean urease inhibitory activities are evaluated. Heating a mixture of 1,1-bis(methylthio)-2-nitroethene, a 1,2-diamine, a 2-formylbenzoic acid and a primary amine in EtOH for 3.5 h afforded the corresponding 2,3-disubstituted isoindolin-1-ones in good to excellent yields. All sixteen synthesized isoindolin-1-one derivatives 5a–p showed urease inhibitory activity. Among them, 5c showed the most urease inhibitory activity (IC50 = 10.07 ± 0.28 µM) being over 2-fold more potent than thiourea (IC50 = 22.01 ± 0.10 µM) and 10-fold than hydroxyurea (IC50 = 100.00 ± 0.02 µM) as the standard inhibitors, respectively. Also, results from molecular docking studies were in good agreement with those obtained from in vitro tests.
中文翻译:

异吲哚啉-1-酮衍生物作为脲酶抑制剂:设计,合成,生物学评估,分子对接和计算机模拟ADME评估。
描述了一个有效的,一锅和四个组成部分的新系列的新的2,3-二取代的异吲哚啉-1-酮的合成,并评估了其对Jack bean脲酶的抑制活性。将1,1-双(甲硫基)-2-硝基乙烯,1,2-二胺,2-甲酰基苯甲酸和伯胺的混合物在EtOH中加热3.5小时,得到相应的2,3-二取代的异吲哚啉-1-产量高到极好的品种。所有16种合成的异吲哚啉-1-酮衍生物5a-p均显示出脲酶抑制活性。其中5c表现出最大的脲酶抑制活性(IC 50 = 10.07±0.28 µM),其效力是硫脲(IC 50 = 22.01±0.10 µM)的2倍以上,是羟基脲(IC 50的10倍) = 100.00±0.02 µM)分别作为标准抑制剂。而且,分子对接研究的结果与体外测试的结果吻合良好。































 京公网安备 11010802027423号
京公网安备 11010802027423号