当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distinguishing Active Site Characteristics of Chlorite Dismutases with Their Cyanide Complexes
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1021/acs.biochem.7b01278 Zachary Geeraerts 1 , Arianna I Celis 2 , Jeffery A Mayfield 3 , Megan Lorenz 1 , Kenton R Rodgers 1 , Jennifer L DuBois 2 , Gudrun S Lukat-Rodgers 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1021/acs.biochem.7b01278 Zachary Geeraerts 1 , Arianna I Celis 2 , Jeffery A Mayfield 3 , Megan Lorenz 1 , Kenton R Rodgers 1 , Jennifer L DuBois 2 , Gudrun S Lukat-Rodgers 1
Affiliation
|
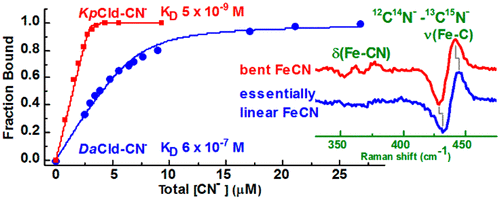
O2-evolving chlorite dismutases (Clds) efficiently convert chlorite (ClO2–) to O2 and Cl–. Dechloromonas aromatica Cld (DaCld) is a highly active chlorite-decomposing homopentameric enzyme, typical of Clds found in perchlorate- and chlorate-respiring bacteria. The Gram-negative, human pathogen Klebsiella pneumoniae contains a homodimeric Cld (KpCld) that also decomposes ClO2–, albeit with an activity 10-fold lower and a turnover number lower than those of DaCld. The interactions between the distal pocket and heme ligand of the DaCld and KpCld active sites have been probed via kinetic, thermodynamic, and spectroscopic behaviors of their cyanide complexes for insight into active site characteristics that are deterministic for chlorite decomposition. At 4.7 × 10–9 M, the KD for the KpCld–CN– complex is 2 orders of magnitude smaller than that of DaCld–CN– and indicates an affinity for CN– that is greater than that of most heme proteins. The difference in CN– affinity between Kp- and DaClds is predominantly due to differences in koff. The kinetics of binding of cyanide to DaCld, DaCld(R183Q), and KpCld between pH 4 and 8.5 corroborate the importance of distal Arg183 and a pKa of ∼7 in stabilizing complexes of anionic ligands, including the substrate. The Fe–C stretching and FeCN bending modes of the DaCld–CN– (νFe–C, 441 cm–1; δFeCN, 396 cm–1) and KpCld–CN– (νFe–C, 441 cm–1; δFeCN, 356 cm–1) complexes reveal differences in their FeCN angle, which suggest different distal pocket interactions with their bound cyanide. Conformational differences in their catalytic sites are also reported by the single ferrous KpCld carbonyl complex, which is in contrast to the two conformers observed for DaCld–CO.
中文翻译:

区分亚氯酸盐歧化酶及其氰化物配合物的活性位点特征
O 2进化亚氯酸盐歧化酶 (Clds) 有效地将亚氯酸盐 (ClO 2 – ) 转化为 O 2和 Cl –。芳香脱氯单胞菌Cld ( Da Cld) 是一种高活性的亚氯酸盐分解同五聚酶,是在高氯酸盐和氯酸盐呼吸细菌中发现的典型 Cld。革兰氏阴性人类病原体肺炎克雷伯菌含有同型二聚体 Cld ( Kp Cld),它也能分解 ClO 2 – ,但活性比Da Cld低 10 倍,周转数也低。通过氰化物复合物的动力学、热力学和光谱行为,探究了Da Cld 和Kp Cld 活性位点的远端口袋和血红素配体之间的相互作用,以深入了解对亚氯酸盐分解具有确定性的活性位点特征。Kp Cld-CN复合物的KD为 4.7 × 10 –9 M,比Da Cld-CN的 KD 小 2个数量级,表明对CN 的亲和力大于大多数血红素蛋白。Kp-和Da Clds 之间CN-亲和力的差异主要是由于k off的差异造成的。pH 4 至 8.5 之间氰化物与Da Cld、Da Cld(R183Q) 和Kp Cld 的结合动力学证实了远端 Arg183 和ap Ka约为 7 在稳定阴离子配体(包括底物)复合物中的重要性。Da Cld–CN – (ν Fe–C , 441 cm –1 ; δ FeCN , 396 cm –1 ) 和Kp Cld–CN – (ν Fe–C , 441 cm –1 )的 Fe–C 拉伸和 FeCN 弯曲模式1 ; δ FeCN , 356 cm –1 ) 配合物揭示了它们的 FeCN 角的差异,这表明远端口袋与其结合的氰化物的相互作用不同。单个亚铁Kp Cld 羰基络合物也报告了它们催化位点的构象差异,这与Da Cld-CO观察到的两个构象异构体形成鲜明对比。
更新日期:2018-02-06
中文翻译:

区分亚氯酸盐歧化酶及其氰化物配合物的活性位点特征
O 2进化亚氯酸盐歧化酶 (Clds) 有效地将亚氯酸盐 (ClO 2 – ) 转化为 O 2和 Cl –。芳香脱氯单胞菌Cld ( Da Cld) 是一种高活性的亚氯酸盐分解同五聚酶,是在高氯酸盐和氯酸盐呼吸细菌中发现的典型 Cld。革兰氏阴性人类病原体肺炎克雷伯菌含有同型二聚体 Cld ( Kp Cld),它也能分解 ClO 2 – ,但活性比Da Cld低 10 倍,周转数也低。通过氰化物复合物的动力学、热力学和光谱行为,探究了Da Cld 和Kp Cld 活性位点的远端口袋和血红素配体之间的相互作用,以深入了解对亚氯酸盐分解具有确定性的活性位点特征。Kp Cld-CN复合物的KD为 4.7 × 10 –9 M,比Da Cld-CN的 KD 小 2个数量级,表明对CN 的亲和力大于大多数血红素蛋白。Kp-和Da Clds 之间CN-亲和力的差异主要是由于k off的差异造成的。pH 4 至 8.5 之间氰化物与Da Cld、Da Cld(R183Q) 和Kp Cld 的结合动力学证实了远端 Arg183 和ap Ka约为 7 在稳定阴离子配体(包括底物)复合物中的重要性。Da Cld–CN – (ν Fe–C , 441 cm –1 ; δ FeCN , 396 cm –1 ) 和Kp Cld–CN – (ν Fe–C , 441 cm –1 )的 Fe–C 拉伸和 FeCN 弯曲模式1 ; δ FeCN , 356 cm –1 ) 配合物揭示了它们的 FeCN 角的差异,这表明远端口袋与其结合的氰化物的相互作用不同。单个亚铁Kp Cld 羰基络合物也报告了它们催化位点的构象差异,这与Da Cld-CO观察到的两个构象异构体形成鲜明对比。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号