当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanisms for Long-Life Rechargeable Zn/MnO2 Batteries
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-02-22 00:00:00 , DOI: 10.1021/acs.chemmater.8b05093 Yun Li 1 , Shanyu Wang 1 , James R. Salvador 2 , Jinpeng Wu 3, 4 , Bo Liu 5 , Wanli Yang 3 , Jiong Yang 5 , Wenqing Zhang 6 , Jun Liu 1, 7 , Jihui Yang 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-02-22 00:00:00 , DOI: 10.1021/acs.chemmater.8b05093 Yun Li 1 , Shanyu Wang 1 , James R. Salvador 2 , Jinpeng Wu 3, 4 , Bo Liu 5 , Wanli Yang 3 , Jiong Yang 5 , Wenqing Zhang 6 , Jun Liu 1, 7 , Jihui Yang 1
Affiliation
|
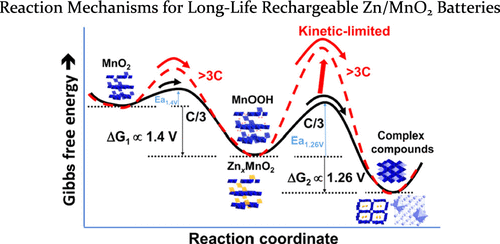
Rechargeable aqueous Zn-ion batteries (ZIBs) are very promising for large-scale grid energy storage applications owing to their low cost, environmentally benign constituents, excellent safety, and relatively high energy density. Their usage, however, is largely hampered by the fast capacity fade. The complexity of the reactions has resulted in long-standing ambiguities of the chemical pathways of Zn/MnO2 system. In this study, we find that both H+/Zn2+ intercalation and conversion reactions occur at different voltages and that the rapid capacity fading can clearly be ascribed to the rate-limiting and irreversible conversion reactions at a lower voltage. By limiting the irreversible conversion reactions at ∼1.26 V, we successfully demonstrate ultrahigh power and long life that are superior to most of the reported ZIBs or even some lithium-ion batteries.
中文翻译:

长寿命可充电Zn / MnO 2电池的反应机理
可再充电的含水锌离子电池(ZIBs)由于其低成本,对环境无害的成分,出色的安全性和相对较高的能量密度,因此在大规模电网储能应用中非常有前途。但是,它们的使用在很大程度上受到快速容量衰减的阻碍。反应的复杂性导致Zn / MnO 2系统的化学路径长期存在歧义。在这项研究中,我们发现H + / Zn 2+插层和转化反应在不同的电压下发生,而快速的容量衰减显然可以归因于较低电压下的限速和不可逆转化反应。通过将不可逆的转化反应限制在〜1.26 V,我们成功证明了超高功率和长寿命,优于大多数报道的ZIB甚至某些锂离子电池。
更新日期:2019-02-22
中文翻译:

长寿命可充电Zn / MnO 2电池的反应机理
可再充电的含水锌离子电池(ZIBs)由于其低成本,对环境无害的成分,出色的安全性和相对较高的能量密度,因此在大规模电网储能应用中非常有前途。但是,它们的使用在很大程度上受到快速容量衰减的阻碍。反应的复杂性导致Zn / MnO 2系统的化学路径长期存在歧义。在这项研究中,我们发现H + / Zn 2+插层和转化反应在不同的电压下发生,而快速的容量衰减显然可以归因于较低电压下的限速和不可逆转化反应。通过将不可逆的转化反应限制在〜1.26 V,我们成功证明了超高功率和长寿命,优于大多数报道的ZIB甚至某些锂离子电池。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号