当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis and Biological Evaluation of Substituted (1‐(4‐chlorobenzyl)‐1H‐indol‐3‐yl) 1H‐(1,2,3‐triazol‐4‐yl)methanones as Antifungal Agents
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-02-22 , DOI: 10.1002/slct.201803572 Mohd Adil Shareef 1, 2 , Hemshikha Rajpurohit 3 , K. Sirisha 3 , Ibrahim Bin Sayeed 3 , Irfan Khan 3 , Manasa Kadagathur 4 , Thipparapu Ganapathi 5 , C. Ganesh Kumar 3 , Ahmed Kamal 6 , Bathini Nagendra Babu 1, 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-02-22 , DOI: 10.1002/slct.201803572 Mohd Adil Shareef 1, 2 , Hemshikha Rajpurohit 3 , K. Sirisha 3 , Ibrahim Bin Sayeed 3 , Irfan Khan 3 , Manasa Kadagathur 4 , Thipparapu Ganapathi 5 , C. Ganesh Kumar 3 , Ahmed Kamal 6 , Bathini Nagendra Babu 1, 2
Affiliation
|
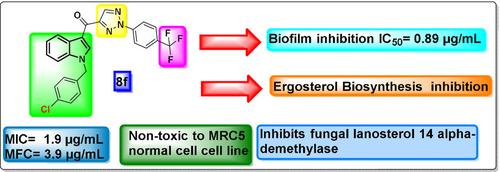
In the perusal of our endeavour towards the synthesis of new bioactive agents, a new series of substituted (1‐(4‐chlorobenzyl)‐1H‐indol‐3‐yl) 1H‐(1,2,3‐triazol‐4‐yl) methanones (8a‐u) were designed, synthesized and investigated for their antimicrobial activity with special emphasis on their anti‐Candida potential and Candida biofilm inhibition. Among them, the hybrids 8e, 8f, and 8o demonstrated significant antifungal activity on most of the tested fungal strains with MIC values ranging between 1.9–7.8 μg/mL. In addition, the most promising compounds were found to be effective biofilm inhibitors. We have studied the Field emission Scanning Electron Microscope (FE‐SEM) micrographs for 8f to know the damage of the cells with altered membrane integrity and release of intracellular contents thereby validating the in vitro biofilm inhibition assay. Further, in enzymatic assay, active compounds inhibited ergosterol biosynthesis. Furthermore, docking studies revealed that they effectively bind to 14α‐demethylase (CYP51), thereby inhibiting ergosterol biosynthesis. Interestingly, they exhibited lower cytotoxicity in the normal cell line (MRC5).
中文翻译:

设计,合成和生物学评估取代的(1-(4-氯苄基)-1H-吲哚-3-基)1H-(1,2,3-三唑-4-基)甲酮作为抗真菌剂
在认真研究合成新的生物活性剂的过程中,出现了一系列新的取代的(1-(4-氯苄基)-1 H-吲哚-3-基)1 H-(1,2,3-三唑-4)设计,合成和研究了甲酰基(yl)甲酮(8a-u)的抗菌活性,并特别强调了其抗念珠菌的潜力和念珠菌对生物膜的抑制作用。其中,混合动力车8e,8f和8o在大多数测试的真菌菌株上均表现出显着的抗真菌活性,MIC值在1.9–7.8μg/ mL之间。另外,发现最有前途的化合物是有效的生物膜抑制剂。我们研究了8f的场发射扫描电子显微镜(FE‐SEM)显微照片,以了解膜完整性改变和细胞内内容物释放而对细胞造成的损害,从而验证了体外生物膜抑制试验的有效性。此外,在酶促测定中,活性化合物抑制了麦角固醇的生物合成。此外,对接研究显示它们可以有效地结合14α-脱甲基酶(CYP51),从而抑制麦角固醇的生物合成。有趣的是,它们在正常细胞系(MRC5)中表现出较低的细胞毒性。
更新日期:2019-02-22
中文翻译:

设计,合成和生物学评估取代的(1-(4-氯苄基)-1H-吲哚-3-基)1H-(1,2,3-三唑-4-基)甲酮作为抗真菌剂
在认真研究合成新的生物活性剂的过程中,出现了一系列新的取代的(1-(4-氯苄基)-1 H-吲哚-3-基)1 H-(1,2,3-三唑-4)设计,合成和研究了甲酰基(yl)甲酮(8a-u)的抗菌活性,并特别强调了其抗念珠菌的潜力和念珠菌对生物膜的抑制作用。其中,混合动力车8e,8f和8o在大多数测试的真菌菌株上均表现出显着的抗真菌活性,MIC值在1.9–7.8μg/ mL之间。另外,发现最有前途的化合物是有效的生物膜抑制剂。我们研究了8f的场发射扫描电子显微镜(FE‐SEM)显微照片,以了解膜完整性改变和细胞内内容物释放而对细胞造成的损害,从而验证了体外生物膜抑制试验的有效性。此外,在酶促测定中,活性化合物抑制了麦角固醇的生物合成。此外,对接研究显示它们可以有效地结合14α-脱甲基酶(CYP51),从而抑制麦角固醇的生物合成。有趣的是,它们在正常细胞系(MRC5)中表现出较低的细胞毒性。








































 京公网安备 11010802027423号
京公网安备 11010802027423号