当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the Mechanism of Cysteine Persulfide Formation Catalyzed by 3-Mercaptopyruvate Sulfurtransferases
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1021/acscatal.7b02432
Jean-Christophe Lec 1 , Séverine Boutserin 1 , Hortense Mazon 1 , Guillermo Mulliert 2 , Sandrine Boschi-Muller 1 , François Talfournier 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1021/acscatal.7b02432
Jean-Christophe Lec 1 , Séverine Boutserin 1 , Hortense Mazon 1 , Guillermo Mulliert 2 , Sandrine Boschi-Muller 1 , François Talfournier 1
Affiliation
|
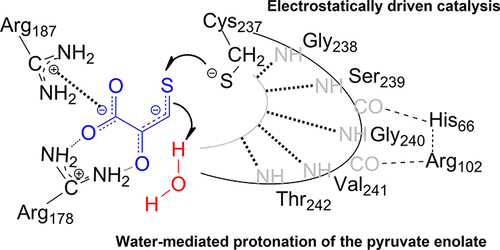
Sulfhydration of reactive cysteines in target proteins is now recognized as a major route by which H2S mediates signal transduction and regulates various cellular processes. Among the enzymatic systems permitting the formation of cysteine persulfide from nonactivated sulfur compounds, 3-mercaptopyruvate sulfurtransferases can be considered as a model of thiolate-based chemistry for carbon–sulfur bond breaking. These ubiquitous enzymes transfer a sulfur atom from 3-mercaptopyruvate (3-MP) to a thiol acceptor via a cysteine-persulfide intermediate, but the mechanistic basis for its formation is still unclear. To address this question, kinetic approaches were developed for studying the reaction catalyzed by the human and Escherichia coli enzymes and the role of several conserved residues was also investigated. We showed that the first step of sulfur transfer that leads to pyruvate release and formation of the persulfide intermediate is very efficient for both enzymes. It critically depends on the electrostatic contribution provided by the CGSGVT catalytic loop, while any role of the so-called Ser/His/Asp triad can be excluded. Furthermore, solvent kinetic isotopic effect and proton inventory studies revealed a concerted mechanism in which the water-mediated protonation of the pyruvate enolate and S0 transfer from the deprotonated 3-MP to the thiolate form of the catalytic cysteine occur concomitantly.
中文翻译:

阐明3-巯基丙酮酸硫转移酶催化的半胱氨酸过硫化物形成的机理
现在,人们认识到靶蛋白中反应性半胱氨酸的硫酸化是H 2 S介导信号转导并调节各种细胞过程的主要途径。在允许由未活化的硫化合物形成半胱氨酸过硫化物的酶系统中,3-巯基丙酮酸硫转移酶可以被认为是基于硫醇盐的碳硫键断裂化学模型。这些普遍存在的酶通过半胱氨酸-过硫化物中间体将硫原子从3-巯基丙酮酸酯(3-MP)转移至硫醇受体,但其形成的机理基础尚不清楚。为了解决这个问题,人们开发了动力学方法来研究人和大肠杆菌催化的反应。还研究了酶和几种保守残基的作用。我们表明,硫转移的第一步导致丙酮酸的释放和过硫化中间体的形成对于两种酶都是非常有效的。它主要取决于CGSGVT催化环提供的静电作用,而所谓的Ser / His / Asp三联体的任何作用都可以排除。此外,溶剂动力学同位素效应和质子库存研究揭示了一种协同机制,其中丙酮酸烯醇盐的水介导质子化和从去质子化的3-MP到催化半胱氨酸的硫醇盐形式的S 0转移同时发生。
更新日期:2018-02-06
中文翻译:

阐明3-巯基丙酮酸硫转移酶催化的半胱氨酸过硫化物形成的机理
现在,人们认识到靶蛋白中反应性半胱氨酸的硫酸化是H 2 S介导信号转导并调节各种细胞过程的主要途径。在允许由未活化的硫化合物形成半胱氨酸过硫化物的酶系统中,3-巯基丙酮酸硫转移酶可以被认为是基于硫醇盐的碳硫键断裂化学模型。这些普遍存在的酶通过半胱氨酸-过硫化物中间体将硫原子从3-巯基丙酮酸酯(3-MP)转移至硫醇受体,但其形成的机理基础尚不清楚。为了解决这个问题,人们开发了动力学方法来研究人和大肠杆菌催化的反应。还研究了酶和几种保守残基的作用。我们表明,硫转移的第一步导致丙酮酸的释放和过硫化中间体的形成对于两种酶都是非常有效的。它主要取决于CGSGVT催化环提供的静电作用,而所谓的Ser / His / Asp三联体的任何作用都可以排除。此外,溶剂动力学同位素效应和质子库存研究揭示了一种协同机制,其中丙酮酸烯醇盐的水介导质子化和从去质子化的3-MP到催化半胱氨酸的硫醇盐形式的S 0转移同时发生。

































 京公网安备 11010802027423号
京公网安备 11010802027423号