当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper‐Catalyzed Annulation/A3‐Coupling Cascade: Diastereodivergent Synthesis of Sterically Hindered Monocyclic Oxazolidines Bearing Multiple Stereocenters
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-02-22 , DOI: 10.1002/ejoc.201900031 Huangdi Feng 1 , Yazhen Zhang 1 , Zedi Zhang 1 , Fubei Chen 1 , Liliang Huang 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-02-22 , DOI: 10.1002/ejoc.201900031 Huangdi Feng 1 , Yazhen Zhang 1 , Zedi Zhang 1 , Fubei Chen 1 , Liliang Huang 1
Affiliation
|
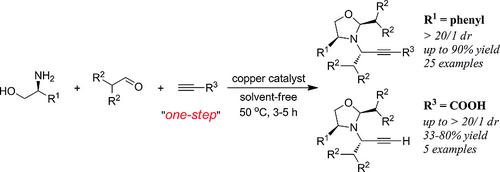
An efficient strategy for the synthesis of sterically hindered chiral N‐propargyl oxazolidines bearing multiple stereocenters through copper‐catalyzed domino A3 reaction has been successfully disclosed. The chiral plenylglycinol precursor allowed the attainment of the target products in good yields (up to 90 %) with excellent diastereomeric ratio (> 20:1). Facile operation in open‐air, solvent‐free, and one‐pot process makes this method practical and attractive.

中文翻译:

铜催化的环形/ A3耦合级联:带有多个立体中心的立体受阻单环恶唑烷的非对映发合成
已经成功地公开了通过铜催化的多米诺骨牌A 3反应合成具有多个立体中心的空间位阻性手性N-炔丙基恶唑烷的有效策略。手性烯基甘氨醇前体允许以优异的非对映体比率(> 20:1)以良好的收率(最高90%)获得目标产物。露天,无溶剂和一锅法的简便操作使该方法实用且有吸引力。

更新日期:2019-02-22

中文翻译:

铜催化的环形/ A3耦合级联:带有多个立体中心的立体受阻单环恶唑烷的非对映发合成
已经成功地公开了通过铜催化的多米诺骨牌A 3反应合成具有多个立体中心的空间位阻性手性N-炔丙基恶唑烷的有效策略。手性烯基甘氨醇前体允许以优异的非对映体比率(> 20:1)以良好的收率(最高90%)获得目标产物。露天,无溶剂和一锅法的简便操作使该方法实用且有吸引力。
































 京公网安备 11010802027423号
京公网安备 11010802027423号