Communications Chemistry ( IF 5.9 ) Pub Date : 2019-01-25 , DOI: 10.1038/s42004-019-0110-y Saima Perveen , Shuang Yang , Miao Meng , Weici Xu , Guoxiang Zhang , Xinqiang Fang
|
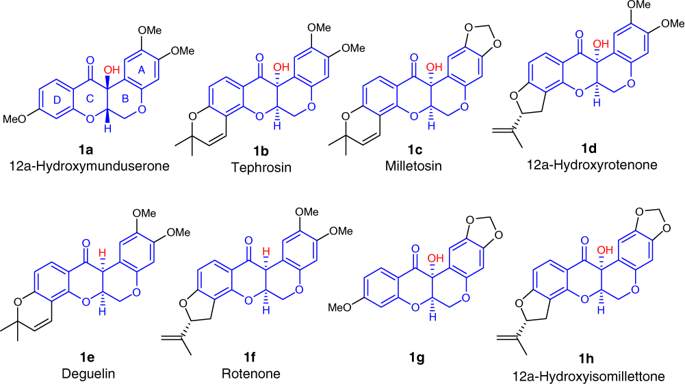
Increasing effort has been made towards the asymmetric total synthesis of rotenoid natural products owing to their impressive biological and pharmaceutical activities. Here we report the modular asymmetric total synthesis of rotenoid natural products. The concise construction of the cis-fused tetrahydrochromeno[3,4-b]chromene core structure of rotenoids through N-heterocyclic carbene-catalyzed dynamic kinetic resolution is achieved, and a series of annulation products containing rotenoid key structures are rapidly assembled using this method. More importantly, the protocol enables the modular synthesis of a variety of rotenoid natural products in a highly convergent fashion, and the concise asymmetric total synthesis of tephrosin, the first asymmetric total synthesis of 12a-hydroxymunduserone, milletosin, and 12a-hydroxyrotenone, and the formal synthesis of deguelin are accomplished.
中文翻译:

类胡萝卜素通过有机催化动态动力学拆分的不对称全合成
由于类胡萝卜素天然产物的令人印象深刻的生物学和药学活性,已经对类胡萝卜素天然产物的不对称全合成进行了越来越多的努力。在这里,我们报告类胡萝卜素天然产物的模块化不对称全合成。顺式的简洁结构通过N-杂环卡宾催化的动态动力学拆分,实现了类胡萝卜素的四氢噻吩并[3,4-b]亚甲基苯并噻吩核结构的熔融,并利用该方法快速组装了一系列包含类胡萝卜素键结构的环合产物。更重要的是,该协议能够以高度收敛的方式模块化合成各种类胡萝卜素天然产物,以及精简的不对称全合成的去氧肾上腺素,第一个不对称的全合成的12a-羟基扁桃酮,小球蛋白和12a-羟基鱼藤酮,以及完成了地精蛋白的正式合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号