iScience ( IF 4.6 ) Pub Date : 2019-02-15 , DOI: 10.1016/j.isci.2019.02.008
Nikita Patel , Juehong Wang , Kumiko Shiozawa , Kevin B. Jones , Yanfeng Zhang , Jeremy W. Prokop , George G. Davenport , Naoe T. Nihira , Zhenyue Hao , Derek Wong , Laurel Brandsmeier , Sarah K. Meadows , Arthur V. Sampaio , Ryan Vander Werff , Makoto Endo , Mario R. Capecchi , Kelly M. McNagny , Tak W. Mak , Torsten O. Nielsen , T. Michael Underhill , Richard M. Myers , Tadashi Kondo , Le Su
|
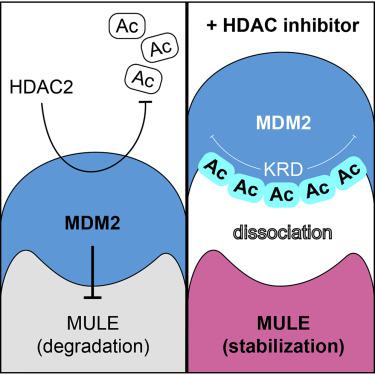
Histone deacetylases (HDACs) are promising targets for cancer therapy, although their individual actions remain incompletely understood. Here, we identify a role for HDAC2 in the regulation of MDM2 acetylation at previously uncharacterized lysines. Upon inactivation of HDAC2, this acetylation creates a structural signal in the lysine-rich domain of MDM2 to prevent the recognition and degradation of its downstream substrate, MCL-1 ubiquitin ligase E3 (MULE). This mechanism further reveals a therapeutic connection between the MULE ubiquitin ligase function and tumor suppression. Specifically, we show that HDAC inhibitor treatment promotes the accumulation of MULE, which diminishes the t(X; 18) translocation-associated synovial sarcomagenesis by directly targeting the fusion product SS18-SSX for degradation. These results uncover a new HDAC2-dependent pathway that integrates reversible acetylation signaling to the anticancer ubiquitin response.
中文翻译:

HDAC2调节MDM2的位点特异性乙酰化及其在肿瘤抑制中的泛素化信号
组蛋白脱乙酰基酶(HDACs)是癌症治疗的有希望的靶标,尽管其各自的作用尚不完全清楚。在这里,我们确定了HDAC2在以前未表征的赖氨酸MDM2乙酰化调节中的作用。HDAC2失活后,这种乙酰化作用会在MDM2的富含赖氨酸的域中产生结构信号,以防止其下游底物MCL-1泛素连接酶E3(MULE)的识别和降解。该机制进一步揭示了MULE泛素连接酶功能与肿瘤抑制之间的治疗联系。具体而言,我们表明,HDAC抑制剂治疗可通过直接靶向融合产物SS18-SSX降解来促进MULE的积累,从而减少t(X; 18)易位相关的滑膜肉瘤的发生。

































 京公网安备 11010802027423号
京公网安备 11010802027423号