当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01788 Nicholas A. Meanwell 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01788 Nicholas A. Meanwell 1
Affiliation
|
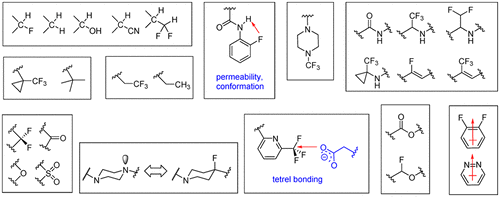
The electronic properties and relatively small size of fluorine endow it with considerable versatility as a bioisostere and it has found application as a substitute for lone pairs of electrons, the hydrogen atom, and the methyl group while also acting as a functional mimetic of the carbonyl, carbinol, and nitrile moieties. In this context, fluorine substitution can influence the potency, conformation, metabolism, membrane permeability, and P-gp recognition of a molecule and temper inhibition of the hERG channel by basic amines. However, as a consequence of the unique properties of fluorine, it features prominently in the design of higher order structural metaphors that are more esoteric in their conception and which reflect a more sophisticated molecular construction that broadens biological mimesis. In this Perspective, applications of fluorine in the construction of bioisosteric elements designed to enhance the in vitro and in vivo properties of a molecule are summarized.
中文翻译:

氟和氟化基序在生物等位基因的设计和药物设计中的应用
氟的电子性质和相对较小的尺寸使其具有很强的通用性,可以作为生物等排物,并且已发现它可以替代电子,氢原子和甲基对的孤对,同时还可以充当羰基的功能模拟物,甲醇和腈基。在这种情况下,氟取代会影响分子的效价,构象,新陈代谢,膜通透性和P-gp识别以及碱性胺对hERG通道的抑制作用。但是,由于氟的独特性质,它在高阶结构隐喻的设计中具有显着特征,这些隐喻的概念更加深奥,并且反映了拓宽生物模仿的更复杂的分子构造。从这个角度来看,
更新日期:2018-02-05
中文翻译:

氟和氟化基序在生物等位基因的设计和药物设计中的应用
氟的电子性质和相对较小的尺寸使其具有很强的通用性,可以作为生物等排物,并且已发现它可以替代电子,氢原子和甲基对的孤对,同时还可以充当羰基的功能模拟物,甲醇和腈基。在这种情况下,氟取代会影响分子的效价,构象,新陈代谢,膜通透性和P-gp识别以及碱性胺对hERG通道的抑制作用。但是,由于氟的独特性质,它在高阶结构隐喻的设计中具有显着特征,这些隐喻的概念更加深奥,并且反映了拓宽生物模仿的更复杂的分子构造。从这个角度来看,






























 京公网安备 11010802027423号
京公网安备 11010802027423号