Communications Chemistry ( IF 5.9 ) Pub Date : 2018-12-03 , DOI: 10.1038/s42004-018-0092-1 Guodu Liu , Wenzhen Fu , Xingye Mu , Ting Wu , Ming Nie , Kaidi Li , Xiaodong Xu , Wenjun Tang
|
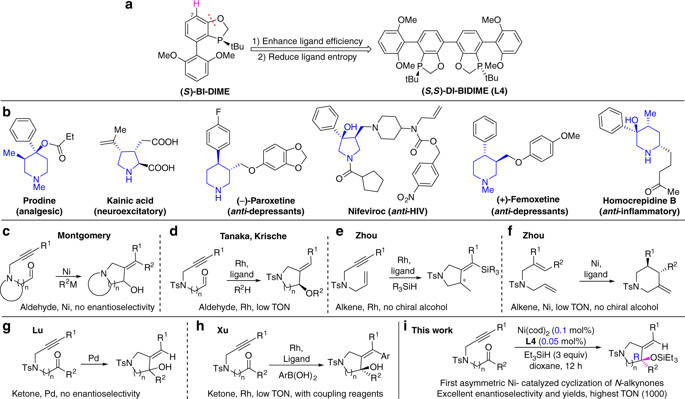
Pyrrolidines and piperidines are important building blocks in organic synthesis. Numerous methods exist for constructing substituted pyrrolidines and piperidines. However, efficient syntheses of pyrrolidines and piperidines bearing chiral tertiary alcohols are limited. Here we report an efficient enantioselective nickel-catalyzed intramolecular reductive cyclization of N-alkynones. A P-chiral bisphosphorus ligand DI-BIDIME is designed and applied in the synthesis of tertiary allylic siloxanes bearing pyrrolidine and piperidine rings in high yields and excellent enantioselectivities, with triethylsilane as reducing reagent. The highest turn over number achieved is 1000 (0.1 mol% catalyst loading) with > 99:1 er. This reaction provides a practical way to synthesize pyrrolidine and piperidine derivatives with chiral tertiary alcohols from easily accessible starting materials under mild conditions. The products can be scaled up and transformed to various useful chiral intermediates. The P-chiral bisphosphorus ligand developed in this study represents one of the few ligands for highly enantioselective cyclization of alkynones.
中文翻译:

镍催化的N-炔酮的对映选择性还原环化,带有手性叔醇的吡咯烷和哌啶
吡咯烷和哌啶是有机合成中的重要组成部分。存在许多用于构建取代的吡咯烷和哌啶的方法。但是,带有手性叔醇的吡咯烷和哌啶的有效合成受到限制。在这里,我们报告了一种有效的对映体选择性镍催化的N分子内还原性环化反应-炔酮。设计了一种P-手性双磷配体DI-BIDIME,并以三乙基硅烷为还原剂,以高收率和优异的对映选择性合成了带有吡咯烷和哌啶环的叔烯丙基硅氧烷。达到的最高周转次数为1000(0.1摩尔%的催化剂负载),且> 99:1 er。该反应提供了一种在温和的条件下由容易获得的起始原料与手性叔醇合成吡咯烷和哌啶衍生物的实用方法。产品可以按比例放大并转化为各种有用的手性中间体。在这项研究中开发的P-手性双磷配体代表了炔烃高度对映选择性环化的少数配体之一。































 京公网安备 11010802027423号
京公网安备 11010802027423号