Communications Chemistry ( IF 5.9 ) Pub Date : 2019-01-04 , DOI: 10.1038/s42004-018-0103-2
I. León , E. R. Alonso , C. Cabezas , S. Mata , J. L. Alonso
|
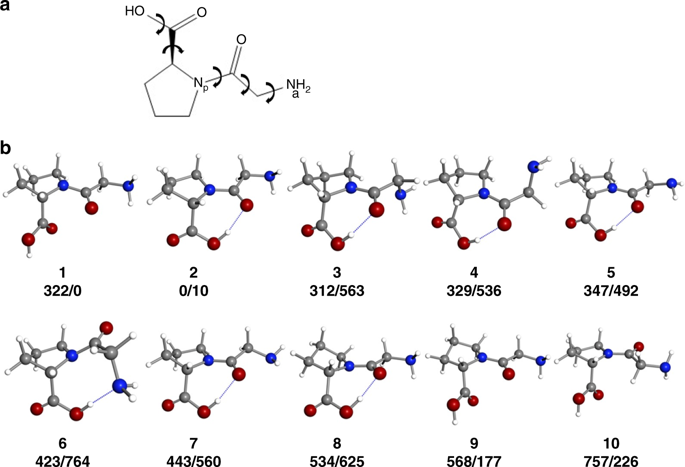
Numerous studies have suggested that the n→π* interactions between carbonyls could contribute significantly to the stability of proteins. Nevertheless, their evaluation is challenging because of the solvent environment or crystal packing forces in solids. Here we study the rotational spectrum of HGlyProOH dipeptide, a very common sequence found in collagen, the most abundant protein in vertebrates, in isolated conditions. Three different structures are unequivocally characterized in the gas phase. Interestingly, the most abundant structure is stabilized by an n→π* interaction and adopts the same conformation as is found in crystalline collagen. This observation serves to support the importance of the n→π* interactions between carbonyl groups.
中文翻译:

揭示二肽中的n→π*相互作用
大量研究表明,羰基之间的n→π*相互作用可显着促进蛋白质的稳定性。然而,由于溶剂环境或固体中的晶体堆积力,它们的评估具有挑战性。在这里,我们研究了HGlyProOH二肽的旋转光谱,该肽是在分离条件下胶原蛋白(脊椎动物中最丰富的蛋白质)中发现的非常常见的序列。气相明确表征了三种不同的结构。有趣的是,最丰富的结构通过n→π*相互作用得以稳定,并具有与结晶胶原蛋白相同的构象。该观察结果支持了羰基之间的n→π*相互作用的重要性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号