iScience ( IF 4.6 ) Pub Date : 2018-12-19 , DOI: 10.1016/j.isci.2018.12.012 Tim Sonntag , Jelena Ostojić , Joan M. Vaughan , James J. Moresco , Young-Sil Yoon , John R. Yates , Marc Montminy
|
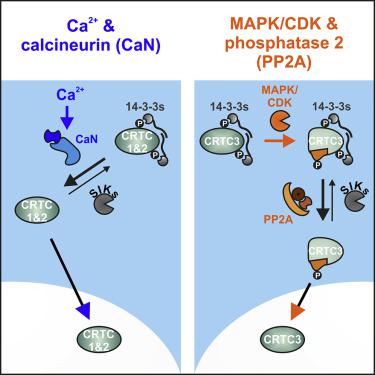
The second messenger 3′,5′-cyclic adenosine monophosphate (cAMP) stimulates gene expression via the cAMP-regulated transcriptional coactivator (CRTC) family of cAMP response element-binding protein coactivators. In the basal state, CRTCs are phosphorylated by salt-inducible kinases (SIKs) and sequestered in the cytoplasm by 14-3-3 proteins. cAMP signaling inhibits the SIKs, leading to CRTC dephosphorylation and nuclear translocation. Here we show that although all CRTCs are regulated by SIKs, their interactions with Ser/Thr-specific protein phosphatases are distinct. CRTC1 and CRTC2 associate selectively with the calcium-dependent phosphatase calcineurin, whereas CRTC3 interacts with B55 PP2A holoenzymes via a conserved PP2A-binding region (amino acids 380–401). CRTC3-PP2A complex formation was induced by phosphorylation of CRTC3 at S391, facilitating the subsequent activation of CRTC3 by dephosphorylation at 14-3-3 binding sites. As stimulation of mitogenic pathways promoted S391 phosphorylation via the activation of ERKs and CDKs, our results demonstrate how a ubiquitous phosphatase enables cross talk between growth factor and cAMP signaling pathways at the level of a transcriptional coactivator.
中文翻译:

有丝分裂信号通过PP2A招聘刺激CREB共激活剂CRTC3。
第二信使3',5'-环磷酸腺苷(cAMP)通过cAMP反应元件结合蛋白共激活因子的cAMP调控转录共激活因子(CRTC)家族刺激基因表达。在基础状态下,CRTC被盐诱导性激酶(SIK)磷酸化,并被14-3-3蛋白隔离在细胞质中。cAMP信号传导抑制SIK,导致CRTC去磷酸化和核易位。在这里,我们显示,尽管所有CRTC均受SIK调控,但它们与Ser / Thr特异性蛋白磷酸酶的相互作用却截然不同。CRTC1和CRTC2与钙依赖性磷酸酶钙调神经磷酸酶选择性结合,而CRTC3通过保守的PP2A结合区(氨基酸380-401)与B55 PP2A全酶相互作用。CRTC3-PP2A复合物的形成是由S391处的CRTC3磷酸化诱导的,通过在14-3-3结合位点进行去磷酸化来促进CRTC3的后续活化。由于促有丝分裂途径的刺激通过ERK和CDK的活化促进了S391的磷酸化,我们的结果证明了普遍存在的磷酸酶如何在转录共激活因子水平上使生长因子和cAMP信号传导途径之间发生串扰。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号