当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Teaching Atom Economy and E-Factor Concepts through a Green Laboratory Experiment: Aerobic Oxidative Cleavage of meso-Hydrobenzoin to Benzaldehyde Using a Heterogeneous Catalyst
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2019-02-20 00:00:00 , DOI: 10.1021/acs.jchemed.8b00058 Chun Ho Lam 1, 2 , Vincent Escande 1 , Karolina E. Mellor 1 , Julie B. Zimmerman 1, 3 , Paul T. Anastas 1, 4
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2019-02-20 00:00:00 , DOI: 10.1021/acs.jchemed.8b00058 Chun Ho Lam 1, 2 , Vincent Escande 1 , Karolina E. Mellor 1 , Julie B. Zimmerman 1, 3 , Paul T. Anastas 1, 4
Affiliation
|
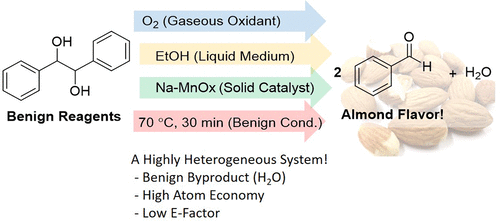
We herein report an efficient aerobic oxidative cleavage of meso-hydrobenzoin to benzaldehyde using a heterogeneous earth-abundant metal oxide catalyst. The reaction can be carried out at 70 °C in ethanol and uses a balloon filled with O2 as oxidant. Reagents are simple and have long shelf lives, and the whole laboratory exercise can be done in 120 min, which makes it highly implementable to standard undergraduate organic curriculum. meso-Hydrobenzoin cleaves exclusively to benzaldehyde, which gives off a nice almond smell, and generates water as the only byproduct. The exercise also compares the present methodology with two other conventional diol oxidative cleavage protocols to illustrate the use of atom economy and E-factor. This laboratory exercise is suitable for second-year undergraduates because it does not require any prerequisites while demonstrating the concepts of catalysis, ease of separation, atom economy, E-factor, earth-abundant material utilization, and biomass transformation.
中文翻译:

通过绿色实验室实验教授原子经济和电子因子概念:使用多相催化剂对内消旋苯并氢分解为苯甲醛进行好氧氧化裂解
我们在本文中报道了使用多相地球富集的金属氧化物催化剂将内消旋-氢安息香有效氧化分解为苯甲醛的方法。该反应可以在70℃的乙醇中进行,并使用装有O 2的气球作为氧化剂。试剂简单,保质期长,整个实验室练习可在120分钟内完成,这使其对标准的本科有机课程具有高度的可实施性。中观-氢安息香专门裂解苯甲醛,散发出良好的杏仁味,并产生水作为唯一的副产物。该练习还将本方法学与其他两个常规的二醇氧化裂解方案进行了比较,以说明原子经济性和E因子的使用。该实验室练习适合二年级本科生,因为它不需要任何先决条件,同时展示了催化,易于分离,原子经济,电子因子,地球上丰富的物质利用和生物质转化的概念。
更新日期:2019-02-20
中文翻译:

通过绿色实验室实验教授原子经济和电子因子概念:使用多相催化剂对内消旋苯并氢分解为苯甲醛进行好氧氧化裂解
我们在本文中报道了使用多相地球富集的金属氧化物催化剂将内消旋-氢安息香有效氧化分解为苯甲醛的方法。该反应可以在70℃的乙醇中进行,并使用装有O 2的气球作为氧化剂。试剂简单,保质期长,整个实验室练习可在120分钟内完成,这使其对标准的本科有机课程具有高度的可实施性。中观-氢安息香专门裂解苯甲醛,散发出良好的杏仁味,并产生水作为唯一的副产物。该练习还将本方法学与其他两个常规的二醇氧化裂解方案进行了比较,以说明原子经济性和E因子的使用。该实验室练习适合二年级本科生,因为它不需要任何先决条件,同时展示了催化,易于分离,原子经济,电子因子,地球上丰富的物质利用和生物质转化的概念。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号