当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triphenylphosphonium-Derived Protein Sulfenic Acid Trapping Agents: Synthesis, Reactivity, and Effect on Mitochondrial Function.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-03-04 , DOI: 10.1021/acs.chemrestox.8b00385 Zhe Li 1 , Tom E Forshaw 2, 3 , Reetta J Holmila 2, 3 , Stephen A Vance 1, 3 , Hanzhi Wu 2, 3 , Leslie B Poole 3, 4 , Cristina M Furdui 2, 3 , S Bruce King 1, 3
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-03-04 , DOI: 10.1021/acs.chemrestox.8b00385 Zhe Li 1 , Tom E Forshaw 2, 3 , Reetta J Holmila 2, 3 , Stephen A Vance 1, 3 , Hanzhi Wu 2, 3 , Leslie B Poole 3, 4 , Cristina M Furdui 2, 3 , S Bruce King 1, 3
Affiliation
|
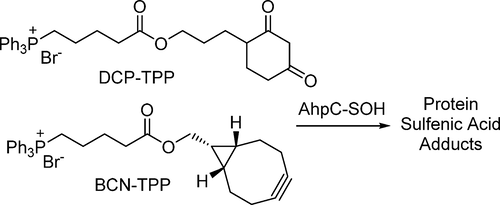
Redox-mediated protein modifications control numerous processes in both normal and disease metabolism. Protein sulfenic acids, formed from the oxidation of protein cysteine residues, play a critical role in thiol-based redox signaling. The reactivity of protein sulfenic acids requires their identification through chemical trapping, and this paper describes the use of the triphenylphosphonium (TPP) ion to direct known sulfenic acid traps to the mitochondria, a verified source of cellular reactive oxygen species. Coupling of the TPP group with the 2,4-(dioxocyclohexyl)propoxy (DCP) unit and the bicyclo[6.1.0]nonyne (BCN) group produces two new probes, DCP-TPP and BCN-TPP. DCP-TPP and BCN-TPP react with C165A AhpC-SOH, a model protein sulfenic acid, to form the expected adducts with second-order rate constants of k = 1.1 M-1 s-1 and k = 5.99 M-1 s-1, respectively, as determined by electrospray ionization time-of-flight mass spectrometry. The TPP group does not alter the rate of DCP-TPP reaction with protein sulfenic acid compared to dimedone but slows the rate of BCN-TPP reaction compared to a non-TPP-containing BCN-OH control by 4.6-fold. The hydrophobic TPP group may interact with the protein, preventing an optimal reaction orientation for BCN-TPP. Unlike BCN-OH, BCN-TPP does not react with the protein persulfide, C165A AhpC-SSH. Extracellular flux measurements using A549 cells show that DCP-TPP and BCN-TPP influence mitochondrial energetics, with BCN-TPP producing a drastic decrease in basal respiration, perhaps due to its faster reaction kinetics with sulfenylated proteins. Further control experiments with BCN-OH, TPP-COOH, and dimedone provide strong evidence for mitochondrial localization and accumulation of DCP-TPP and BCN-TPP. These results reveal the compatibility of the TPP group with reactive sulfenic acid probes as a mitochondrial director and support the use of the TPP group in the design of sulfenic acid traps.
中文翻译:

三苯基phosph衍生的蛋白质亚磺酸捕获剂:合成,反应性和对线粒体功能的影响。
氧化还原介导的蛋白质修饰控制着正常和疾病代谢中的许多过程。由蛋白质半胱氨酸残基的氧化形成的蛋白质亚磺酸在基于硫醇的氧化还原信号中起关键作用。蛋白质亚磺酸的反应性需要通过化学捕集对其进行鉴定,本文描述了使用三苯基phosph(TPP)离子将已知的亚磺酸捕集器引导至线粒体,线粒体是一种经过验证的细胞活性氧物质。TPP基团与2,4-(二氧杂环己基)丙氧基(DCP)单元和双环[6.1.0] nonyne(BCN)基团的偶联产生了两个新的探针DCP-TPP和BCN-TPP。DCP-TPP和BCN-TPP与C165A AhpC-SOH(一种模型蛋白质亚磺酸)反应,形成预期的加合物,其二级速率常数为k = 1.1 M-1 s-1和k = 5.99 M-1 s- 1,分别由电喷雾电离飞行时间质谱测定。与二甲酮相比,TPP组不会改变与蛋白质亚磺酸的DCP-TPP反应速率,但与不含TPP的BCN-OH对照相比,BCN-TPP反应速率却减慢了4.6倍。疏水的TPP基团可能与蛋白质相互作用,从而阻止了BCN-TPP的最佳反应方向。与BCN-OH不同,BCN-TPP不与过硫化蛋白C165A AhpC-SSH反应。使用A549细胞进行的细胞外通量测量表明,DCP-TPP和BCN-TPP影响线粒体的能量,BCN-TPP导致基础呼吸的急剧下降,这可能是由于其与亚磺酰化蛋白的反应动力学更快。使用BCN-OH,TPP-COOH进行进一步的对照实验,和二甲酮为DCP-TPP和BCN-TPP的线粒体定位和积累提供了有力的证据。这些结果揭示了TPP基团与作为线粒体导向剂的反应性磺酸探针的相容性,并支持了TPP基团在次硫酸捕获器设计中的使用。
更新日期:2019-02-20
中文翻译:

三苯基phosph衍生的蛋白质亚磺酸捕获剂:合成,反应性和对线粒体功能的影响。
氧化还原介导的蛋白质修饰控制着正常和疾病代谢中的许多过程。由蛋白质半胱氨酸残基的氧化形成的蛋白质亚磺酸在基于硫醇的氧化还原信号中起关键作用。蛋白质亚磺酸的反应性需要通过化学捕集对其进行鉴定,本文描述了使用三苯基phosph(TPP)离子将已知的亚磺酸捕集器引导至线粒体,线粒体是一种经过验证的细胞活性氧物质。TPP基团与2,4-(二氧杂环己基)丙氧基(DCP)单元和双环[6.1.0] nonyne(BCN)基团的偶联产生了两个新的探针DCP-TPP和BCN-TPP。DCP-TPP和BCN-TPP与C165A AhpC-SOH(一种模型蛋白质亚磺酸)反应,形成预期的加合物,其二级速率常数为k = 1.1 M-1 s-1和k = 5.99 M-1 s- 1,分别由电喷雾电离飞行时间质谱测定。与二甲酮相比,TPP组不会改变与蛋白质亚磺酸的DCP-TPP反应速率,但与不含TPP的BCN-OH对照相比,BCN-TPP反应速率却减慢了4.6倍。疏水的TPP基团可能与蛋白质相互作用,从而阻止了BCN-TPP的最佳反应方向。与BCN-OH不同,BCN-TPP不与过硫化蛋白C165A AhpC-SSH反应。使用A549细胞进行的细胞外通量测量表明,DCP-TPP和BCN-TPP影响线粒体的能量,BCN-TPP导致基础呼吸的急剧下降,这可能是由于其与亚磺酰化蛋白的反应动力学更快。使用BCN-OH,TPP-COOH进行进一步的对照实验,和二甲酮为DCP-TPP和BCN-TPP的线粒体定位和积累提供了有力的证据。这些结果揭示了TPP基团与作为线粒体导向剂的反应性磺酸探针的相容性,并支持了TPP基团在次硫酸捕获器设计中的使用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号