当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-02-20 , DOI: 10.1038/s41419-018-1271-9
Hailong Qi 1, 2, 3 , Xianling Ning 1, 2, 4 , Chang Yu 1, 2, 4 , Xin Ji 1, 2, 4 , Yan Jin 1, 4 , Michael A McNutt 1, 4 , Yuxin Yin 1, 2, 3, 4
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-02-20 , DOI: 10.1038/s41419-018-1271-9
Hailong Qi 1, 2, 3 , Xianling Ning 1, 2, 4 , Chang Yu 1, 2, 4 , Xin Ji 1, 2, 4 , Yan Jin 1, 4 , Michael A McNutt 1, 4 , Yuxin Yin 1, 2, 3, 4
Affiliation
|
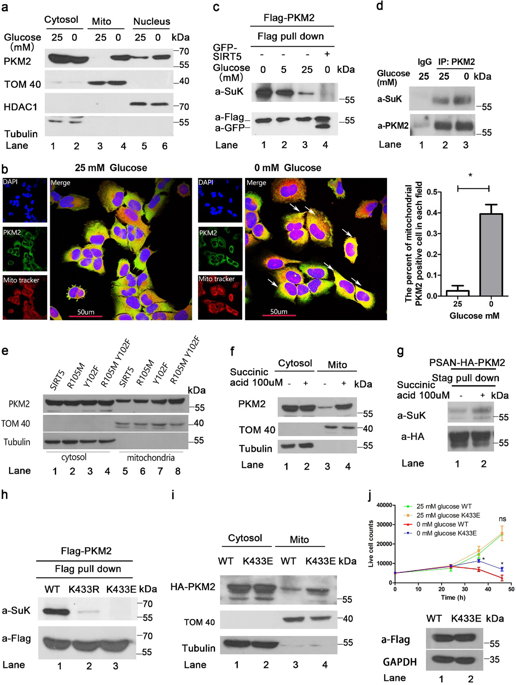
Tumor growth and progression is characteristically associated with the synergistic effects of uncontrolled cellular proliferation and cell survival under stress. Pyruvate kinase M2 (PKM2) contributes to both of these effects. However, the specific mechanism by which PKM2 promotes uncontrolled proliferation or cell survival under stress in different nutritional environments is unclear. We show that succinylation mediated mitochondrial translocation of PKM2 under glucose starvation plays a role in switching the cellular machinery from proliferation to cell survival mode and vice versa. Mitochondrial PKM2 inhibits ubiquitination-mediated degradation of voltage-dependent anion channel 3 (VDAC3) and increases mitochondrial permeability to generate more ATP for cell survival under nutritional depletion. We found there is a positive correlation of upregulation of mitochondrial PKM2 and upregulation of VDAC3 in human colon cancer. This shows the mechanisms identified in this study in fact play a role in neoplastic biology. We therefore developed a small molecule designated compound 8 that blocks mitochondrial translocation of PKM2 and inhibits tumor development. Our data suggest that blocking PKM2 mitochondrial function with a small molecule inhibitor has potential for cancer treatment.
中文翻译:

依赖于琥珀酰化的线粒体PKM2易位,可响应营养应激而促进细胞存活。
肿瘤的生长和发展特征性地与不受控制的细胞增殖和应激下细胞存活的协同作用有关。丙酮酸激酶M2(PKM2)有助于这两种作用。但是,在不同营养环境中,PKM2在压力下促进不受控制的增殖或细胞存活的具体机制尚不清楚。我们显示,在葡萄糖饥饿下,琥珀酸介导的PKM2的线粒体易位在将细胞机制从增殖模式切换到细胞生存模式方面起着作用,反之亦然。线粒体PKM2抑制泛素介导的电压依赖性阴离子通道3(VDAC3)降解,并增加线粒体通透性,从而在营养耗竭下为细胞存活提供更多的ATP。我们发现人结肠癌中线粒体PKM2的上调与VDAC3的上调呈正相关。这表明本研究中确定的机制实际上在肿瘤生物学中起作用。因此,我们开发了一种名为化合物8的小分子,该化合物可阻止PKM2的线粒体易位并抑制肿瘤的发展。我们的数据表明,用小分子抑制剂阻断PKM2线粒体功能具有治疗癌症的潜力。
更新日期:2019-02-20
中文翻译:

依赖于琥珀酰化的线粒体PKM2易位,可响应营养应激而促进细胞存活。
肿瘤的生长和发展特征性地与不受控制的细胞增殖和应激下细胞存活的协同作用有关。丙酮酸激酶M2(PKM2)有助于这两种作用。但是,在不同营养环境中,PKM2在压力下促进不受控制的增殖或细胞存活的具体机制尚不清楚。我们显示,在葡萄糖饥饿下,琥珀酸介导的PKM2的线粒体易位在将细胞机制从增殖模式切换到细胞生存模式方面起着作用,反之亦然。线粒体PKM2抑制泛素介导的电压依赖性阴离子通道3(VDAC3)降解,并增加线粒体通透性,从而在营养耗竭下为细胞存活提供更多的ATP。我们发现人结肠癌中线粒体PKM2的上调与VDAC3的上调呈正相关。这表明本研究中确定的机制实际上在肿瘤生物学中起作用。因此,我们开发了一种名为化合物8的小分子,该化合物可阻止PKM2的线粒体易位并抑制肿瘤的发展。我们的数据表明,用小分子抑制剂阻断PKM2线粒体功能具有治疗癌症的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号