当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A phase 1b/2, open-label, dose-escalation, and dose-confirmation study of eribulin mesilate in combination with capecitabine.
British Journal of Cancer ( IF 6.4 ) Pub Date : 2019-02-20 , DOI: 10.1038/s41416-018-0366-5 Chris Twelves 1 , Alan Anthoney 1 , Claudio I Savulsky 2 , Matthew Guo 3 , Larisa Reyderman 4 , Nicola Cresti 5 , Vladimir Semiglazov 6 , Constanta Timcheva 7 , Ishtiaq Zubairi 8 , Rosemary Morrison 9 , Ruth Plummer 5 , T R Jeffry Evans 9, 10
British Journal of Cancer ( IF 6.4 ) Pub Date : 2019-02-20 , DOI: 10.1038/s41416-018-0366-5 Chris Twelves 1 , Alan Anthoney 1 , Claudio I Savulsky 2 , Matthew Guo 3 , Larisa Reyderman 4 , Nicola Cresti 5 , Vladimir Semiglazov 6 , Constanta Timcheva 7 , Ishtiaq Zubairi 8 , Rosemary Morrison 9 , Ruth Plummer 5 , T R Jeffry Evans 9, 10
Affiliation
|
BACKGROUND
Capecitabine and eribulin are widely used as single agents in metastatic breast cancer (MBC) and have nonoverlapping toxicities.
METHODS
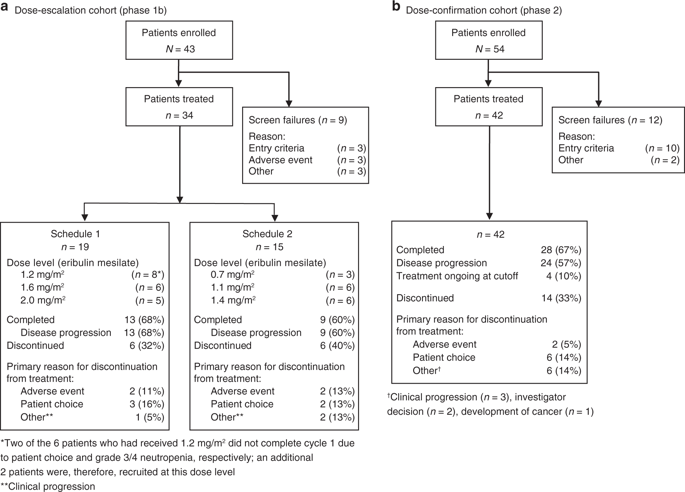
In phase 1b (dose escalation), patients with advanced, treatment-refractory, solid tumours received eribulin mesilate intravenously in 21-day cycles according to schedule 1 (day 1) or schedule 2 (days 1, 8) with twice-daily oral capecitabine (1000 mg/m2 days 1-14). In phase 2 (dose confirmation), women with advanced/MBC and ≤3 prior chemotherapies received eribulin mesilate at the maximum tolerated dose (MTD) per the preferred schedule plus capecitabine. Primary objectives were MTD and dose-limiting toxicities (DLTs; phase 1b) and objective response rate (ORR; phase 2). Secondary objectives included progression-free survival (PFS), safety, and pharmacokinetics.
RESULTS
DLTs occurred in 4/19 patients (schedule 1) and 2/15 patients (schedule 2). Eribulin pharmacokinetics were dose proportional, irrespective of schedule or capecitabine coadministration. The MTD of eribulin was 1.6 mg/m2 day 1 for schedule 1 and 1.4 mg/m2 days 1 and 8 for schedule 2. ORR in phase 2 (eribulin 1.4 mg/m2 days 1, 8 plus capecitabine) was 43% and median PFS 7.2 months. The most common treatment-related adverse events were neutropenia, leukopenia, alopecia, nausea, and lethargy.
CONCLUSIONS
The combination of capecitabine and eribulin showed promising efficacy with manageable tolerability in patients with MBC.
中文翻译:

甲磺酸艾日布林联合卡培他滨的 1b/2 期、开放标签、剂量递增和剂量确认研究。
背景卡培他滨和艾日布林作为单一药物广泛用于转移性乳腺癌(MBC)并且具有非重叠毒性。方法 在 1b 期(剂量递增)中,晚期难治性实体瘤患者根据方案 1(第 1 天)或方案 2(第 1、8 天)以 21 天为一个周期,静脉注射甲磺酸艾日布林,每日两次口服卡培他滨(1000 mg/m2,第 1-14 天)。在第 2 阶段(剂量确认)中,患有晚期/MBC 且既往化疗次数≤3 次的女性按照首选方案接受最大耐受剂量 (MTD) 的甲磺酸艾日布林加卡培他滨。主要目标是 MTD 和剂量限制毒性(DLT;1b 期)和客观缓解率(ORR;2 期)。次要目标包括无进展生存期 (PFS)、安全性和药代动力学。结果 4/19 名患者(附表 1)和 2/15 名患者(附表 2)发生 DLT。艾日布林药代动力学与剂量成正比,与给药方案或卡培他滨联合给药无关。方案 1 的艾日布林的 MTD 为第 1 天 1.6 mg/m2,方案 2 的第 1 天和第 8 天为 1.4 mg/m2。第 2 期(艾日布林 1.4 mg/m2 第 1、8 天加卡培他滨)的 ORR 为 43%,中位 PFS 7.2个月。最常见的治疗相关不良事件是中性粒细胞减少、白细胞减少、脱发、恶心和嗜睡。结论 卡培他滨和艾日布林联合治疗 MBC 患者显示出良好的疗效和可控的耐受性。
更新日期:2019-02-20
中文翻译:

甲磺酸艾日布林联合卡培他滨的 1b/2 期、开放标签、剂量递增和剂量确认研究。
背景卡培他滨和艾日布林作为单一药物广泛用于转移性乳腺癌(MBC)并且具有非重叠毒性。方法 在 1b 期(剂量递增)中,晚期难治性实体瘤患者根据方案 1(第 1 天)或方案 2(第 1、8 天)以 21 天为一个周期,静脉注射甲磺酸艾日布林,每日两次口服卡培他滨(1000 mg/m2,第 1-14 天)。在第 2 阶段(剂量确认)中,患有晚期/MBC 且既往化疗次数≤3 次的女性按照首选方案接受最大耐受剂量 (MTD) 的甲磺酸艾日布林加卡培他滨。主要目标是 MTD 和剂量限制毒性(DLT;1b 期)和客观缓解率(ORR;2 期)。次要目标包括无进展生存期 (PFS)、安全性和药代动力学。结果 4/19 名患者(附表 1)和 2/15 名患者(附表 2)发生 DLT。艾日布林药代动力学与剂量成正比,与给药方案或卡培他滨联合给药无关。方案 1 的艾日布林的 MTD 为第 1 天 1.6 mg/m2,方案 2 的第 1 天和第 8 天为 1.4 mg/m2。第 2 期(艾日布林 1.4 mg/m2 第 1、8 天加卡培他滨)的 ORR 为 43%,中位 PFS 7.2个月。最常见的治疗相关不良事件是中性粒细胞减少、白细胞减少、脱发、恶心和嗜睡。结论 卡培他滨和艾日布林联合治疗 MBC 患者显示出良好的疗效和可控的耐受性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号