当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Helix-Sense-Selective and Enantiomer-Selective Living Polymerization of Phenyl Isocyanide Induced by Reusable Chiral Lactide Using Achiral Palladium Initiator
Macromolecules ( IF 5.1 ) Pub Date : 2015-10-28 00:00:00 , DOI: 10.1021/acs.macromol.5b02124 Jia-Li Chen 1 , Li Yang 1 , Qian Wang 1 , Zhi-Qiang Jiang 1 , Na Liu 1 , Jun Yin 1 , Yunsheng Ding 1 , Zong-Quan Wu 1
Macromolecules ( IF 5.1 ) Pub Date : 2015-10-28 00:00:00 , DOI: 10.1021/acs.macromol.5b02124 Jia-Li Chen 1 , Li Yang 1 , Qian Wang 1 , Zhi-Qiang Jiang 1 , Na Liu 1 , Jun Yin 1 , Yunsheng Ding 1 , Zong-Quan Wu 1
Affiliation
|
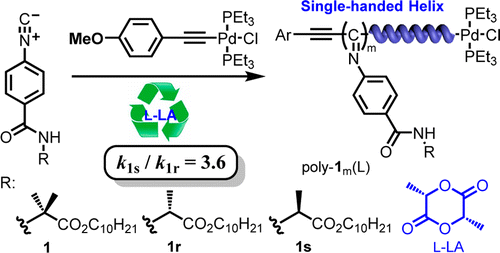
Polymerization of phenyl isocyanide using achiral Pd(II) initiator with the presence of chiral l- or d-lactide (l-LA or d-LA) as additive was found to proceed in helix-sense-selective manner. The polymerization of achiral phenyl isocyanide, 4-isocyanobenzoyl-2-aminoisobutyric acid decyl ester (1) by this method produced optically active helical poly-1m(L), whose chirality was solely come from the helical conformation without containing of any other chiral atoms. The added chiral LA can be facilely recovered and reused in the helix-sense-selective polymerizations without significantly loss of its chiral induction, and the chiral economy of the polymerization is high. When enantiomerically pure phenyl isocyanide bearing an R- or S-alanine pendent with a long n-decyl chain (1r or 1s) were polymerized by this method, the polymerization was found to proceed in a highly enantiomer-selective manner with one of the enantiomers preferentially polymerized over the antipode by a factor of 3.6. Single-handed helical polyisocyanides can be achieved when the chirality of the monomer was appropriately matched with the added LA.
中文翻译:

使用手性钯引发剂的可重复使用的手性丙交酯诱导的苯基异氰酸酯的螺旋义选择性和对映异构体选择性活性聚合
发现使用非手性Pd(II)引发剂在手性1-或d-丙交酯(1 -LA或d -LA)作为添加剂存在下的苯基异氰化物的聚合以螺旋-感觉-选择性方式进行。通过该方法聚合非手性苯基异氰,4-异氰基苯甲酰基-2-氨基异丁酸癸酯(1),可制得旋光性螺旋聚1 m(L),其手性仅来自螺旋构象而不含任何其他手性原子。所添加的手性LA可以容易地回收并在螺旋-感觉-选择性聚合中重复使用,而不会显着损失其手性诱导,并且聚合的手性经济性高。当对映体纯的带有R-或S-丙氨酸侧基长n-癸基链(1r或1s)通过这种方法聚合,发现聚合反应以高度对映异构体选择性的方式进行,其中一种对映异构体优先于对映体聚合3.6倍。当单体的手性与添加的LA适当匹配时,可以实现单手螺旋聚异氰酸酯。
更新日期:2015-10-28
中文翻译:

使用手性钯引发剂的可重复使用的手性丙交酯诱导的苯基异氰酸酯的螺旋义选择性和对映异构体选择性活性聚合
发现使用非手性Pd(II)引发剂在手性1-或d-丙交酯(1 -LA或d -LA)作为添加剂存在下的苯基异氰化物的聚合以螺旋-感觉-选择性方式进行。通过该方法聚合非手性苯基异氰,4-异氰基苯甲酰基-2-氨基异丁酸癸酯(1),可制得旋光性螺旋聚1 m(L),其手性仅来自螺旋构象而不含任何其他手性原子。所添加的手性LA可以容易地回收并在螺旋-感觉-选择性聚合中重复使用,而不会显着损失其手性诱导,并且聚合的手性经济性高。当对映体纯的带有R-或S-丙氨酸侧基长n-癸基链(1r或1s)通过这种方法聚合,发现聚合反应以高度对映异构体选择性的方式进行,其中一种对映异构体优先于对映体聚合3.6倍。当单体的手性与添加的LA适当匹配时,可以实现单手螺旋聚异氰酸酯。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号