当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 2-(6-(5-Chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide (PF-06282999): A Highly Selective Mechanism-Based Myeloperoxidase Inhibitor for the Treatment of Cardiovascular Diseases
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-10-28 00:00:00 , DOI: 10.1021/acs.jmedchem.5b00963 Roger B. Ruggeri 1 , Leonard Buckbinder 1 , Scott W. Bagley 1 , Philip A. Carpino 1 , Edward L. Conn 1 , Matthew S. Dowling 1 , Dilinie P. Fernando 1 , Wenhua Jiao 1 , Daniel W. Kung 1 , Suvi T. M. Orr 1 , Yingmei Qi 1 , Benjamin N. Rocke 1 , Aaron Smith 1 , Joseph S. Warmus 1 , Yan Zhang 1 , Daniel Bowles 1 , Daniel W. Widlicka 1 , Heather Eng 1 , Tim Ryder 1 , Raman Sharma 1 , Angela Wolford 1 , Carlin Okerberg 1 , Karen Walters 1 , Tristan S. Maurer 1 , Yanwei Zhang 1 , Paul D. Bonin 1 , Samantha N. Spath 1 , Gang Xing 1 , David Hepworth 1 , Kay Ahn 1 , Amit S. Kalgutkar 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-10-28 00:00:00 , DOI: 10.1021/acs.jmedchem.5b00963 Roger B. Ruggeri 1 , Leonard Buckbinder 1 , Scott W. Bagley 1 , Philip A. Carpino 1 , Edward L. Conn 1 , Matthew S. Dowling 1 , Dilinie P. Fernando 1 , Wenhua Jiao 1 , Daniel W. Kung 1 , Suvi T. M. Orr 1 , Yingmei Qi 1 , Benjamin N. Rocke 1 , Aaron Smith 1 , Joseph S. Warmus 1 , Yan Zhang 1 , Daniel Bowles 1 , Daniel W. Widlicka 1 , Heather Eng 1 , Tim Ryder 1 , Raman Sharma 1 , Angela Wolford 1 , Carlin Okerberg 1 , Karen Walters 1 , Tristan S. Maurer 1 , Yanwei Zhang 1 , Paul D. Bonin 1 , Samantha N. Spath 1 , Gang Xing 1 , David Hepworth 1 , Kay Ahn 1 , Amit S. Kalgutkar 1
Affiliation
|
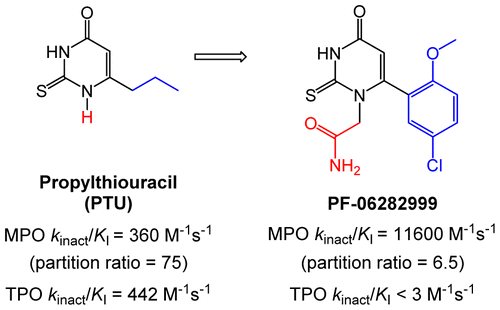
Myeloperoxidase (MPO) is a heme peroxidase that catalyzes the production of hypochlorous acid. Clinical evidence suggests a causal role for MPO in various autoimmune and inflammatory disorders including vasculitis and cardiovascular and Parkinson’s diseases, implying that MPO inhibitors may represent a therapeutic treatment option. Herein, we present the design, synthesis, and preclinical evaluation of N1-substituted-6-arylthiouracils as potent and selective inhibitors of MPO. Inhibition proceeded in a time-dependent manner by a covalent, irreversible mechanism, which was dependent upon MPO catalysis, consistent with mechanism-based inactivation. N1-Substituted-6-arylthiouracils exhibited low partition ratios and high selectivity for MPO over thyroid peroxidase and cytochrome P450 isoforms. N1-Substituted-6-arylthiouracils also demonstrated inhibition of MPO activity in lipopolysaccharide-stimulated human whole blood. Robust inhibition of plasma MPO activity was demonstrated with the lead compound 2-(6-(5-chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide (PF-06282999, 8) upon oral administration to lipopolysaccharide-treated cynomolgus monkeys. On the basis of its pharmacological and pharmacokinetic profile, PF-06282999 has been advanced to first-in-human pharmacokinetic and safety studies.
中文翻译:

2-(6-(5-氯-2-甲氧基苯基)-4-氧代-2-硫代氧代3,4-二氢嘧啶-1(2 H)-基)乙酰胺(PF-06282999)的发现:高度选择性的机制基的髓过氧化物酶抑制剂治疗心血管疾病
髓过氧化物酶(MPO)是一种血红素过氧化物酶,可催化次氯酸的产生。临床证据表明,MPO在各种自身免疫和炎性疾病(包括血管炎,心血管疾病和帕金森氏病)中具有因果作用,这表明MPO抑制剂可能代表了治疗选择。本文中,我们介绍了N -1取代的6-芳基硫尿嘧啶作为MPO的有效抑制剂和选择性抑制剂的设计,合成和临床前评价。抑制作用是通过时间依赖性的方式通过共价不可逆的机制进行的,该机制依赖于MPO催化,与基于机制的失活相一致。ñ与甲状腺过氧化物酶和细胞色素P450同工型相比,1-取代的6-芳基硫尿嘧啶对MPO的分配率低且选择性高。在脂多糖刺激的人全血中,N 1-取代的6-芳基硫尿嘧啶也显示出MPO活性的抑制作用。铅化合物2-(6-(5-氯-2-甲氧基苯基)-4-氧代-2-硫代氧代-3,4-二氢嘧啶-1(2 H)-基)乙酰胺证明了对血浆MPO活性的强烈抑制作用。(PF-06282999,8)口服给药时,以脂多糖处理的猕猴。基于其药理和药代动力学特征,PF-06282999已被推进到人体内药代动力学和安全性研究中。
更新日期:2015-10-28
中文翻译:

2-(6-(5-氯-2-甲氧基苯基)-4-氧代-2-硫代氧代3,4-二氢嘧啶-1(2 H)-基)乙酰胺(PF-06282999)的发现:高度选择性的机制基的髓过氧化物酶抑制剂治疗心血管疾病
髓过氧化物酶(MPO)是一种血红素过氧化物酶,可催化次氯酸的产生。临床证据表明,MPO在各种自身免疫和炎性疾病(包括血管炎,心血管疾病和帕金森氏病)中具有因果作用,这表明MPO抑制剂可能代表了治疗选择。本文中,我们介绍了N -1取代的6-芳基硫尿嘧啶作为MPO的有效抑制剂和选择性抑制剂的设计,合成和临床前评价。抑制作用是通过时间依赖性的方式通过共价不可逆的机制进行的,该机制依赖于MPO催化,与基于机制的失活相一致。ñ与甲状腺过氧化物酶和细胞色素P450同工型相比,1-取代的6-芳基硫尿嘧啶对MPO的分配率低且选择性高。在脂多糖刺激的人全血中,N 1-取代的6-芳基硫尿嘧啶也显示出MPO活性的抑制作用。铅化合物2-(6-(5-氯-2-甲氧基苯基)-4-氧代-2-硫代氧代-3,4-二氢嘧啶-1(2 H)-基)乙酰胺证明了对血浆MPO活性的强烈抑制作用。(PF-06282999,8)口服给药时,以脂多糖处理的猕猴。基于其药理和药代动力学特征,PF-06282999已被推进到人体内药代动力学和安全性研究中。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号