当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antifreeze glycoproteins bind reversibly to ice via hydrophobic groups
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-02 , DOI: 10.1021/jacs.7b13630 Kenji Mochizuki 1, 2 , Valeria Molinero 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-02 , DOI: 10.1021/jacs.7b13630 Kenji Mochizuki 1, 2 , Valeria Molinero 1
Affiliation
|
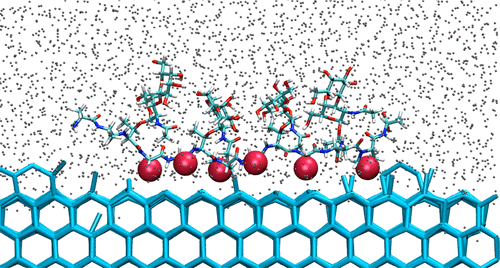
Antifreeze molecules allow organisms to survive in subzero environments. Antifreeze glycoproteins (AFGPs), produced by polar fish, are the most potent inhibitors of ice recrystallization. To date, the molecular mechanism by which AFGPs bind to ice has not yet been elucidated. Mutation experiments cannot resolve whether the binding occurs through the peptide, the saccharides, or both. Here, we use molecular simulations to determine the mechanism and driving forces for binding of AFGP8 to ice, its selectivity for the primary prismatic plane, and the molecular origin of its exceptional ice recrystallization activity. Consistent with experiments, AFGP8 in simulations preferentially adopts the PPII helix secondary structure in solution. We show that the segregation of hydrophilic and hydrophobic groups in the PPII helix is vital for ice binding. Binding occurs through adsorption of methyl groups of the peptide and disaccharides to ice, driven by the entropy of dehydration of the hydrophobic groups as they nest in the cavities at the ice surface. The selectivity to the primary prismatic plane originates in the deeper cavities it has compared to the basal plane. We estimate the free energy of binding of AFGP8 and the longer AFGPs4-6, and find them to be consistent with the reversible binding demonstrated in experiments. The simulations reveal that AFGP8 binds to ice through a myriad of conformations that it uses to diffuse through the ice surface and find ice steps, to which it strongly adsorbs. We interpret that the existence of multiple, weak binding sites is the key for the exceptional ice recrystallization inhibition activity of AFGPs.
中文翻译:

抗冻糖蛋白通过疏水基团与冰可逆结合
防冻分子使生物体能够在零度以下的环境中生存。极地鱼类产生的抗冻糖蛋白 (AFGP) 是冰重结晶的最有效抑制剂。迄今为止,AFGPs 与冰结合的分子机制尚未阐明。突变实验无法解决结合是通过肽、糖类还是两者都发生的。在这里,我们使用分子模拟来确定 AFGP8 与冰结合的机制和驱动力、其对主棱柱面的选择性以及其特殊冰重结晶活动的分子起源。与实验一致,模拟中的 AFGP8 在溶液中优先采用 PPII 螺旋二级结构。我们表明 PPII 螺旋中亲水和疏水基团的分离对于冰结合至关重要。结合是通过肽和二糖的甲基基团吸附到冰上而发生的,这是由疏水基团在它们嵌套在冰表面的空腔中时的脱水熵驱动的。对主棱柱面的选择性源于它与基面相比具有更深的空腔。我们估计了 AFGP8 和更长的 AFGPs4-6 结合的自由能,发现它们与实验中证明的可逆结合一致。模拟结果表明,AFGP8 通过无数构象与冰结合,这些构象用于扩散穿过冰面并找到冰阶,并强烈吸附在冰阶上。我们解释说,多个弱结合位点的存在是 AFGP 异常冰重结晶抑制活性的关键。
更新日期:2018-02-02
中文翻译:

抗冻糖蛋白通过疏水基团与冰可逆结合
防冻分子使生物体能够在零度以下的环境中生存。极地鱼类产生的抗冻糖蛋白 (AFGP) 是冰重结晶的最有效抑制剂。迄今为止,AFGPs 与冰结合的分子机制尚未阐明。突变实验无法解决结合是通过肽、糖类还是两者都发生的。在这里,我们使用分子模拟来确定 AFGP8 与冰结合的机制和驱动力、其对主棱柱面的选择性以及其特殊冰重结晶活动的分子起源。与实验一致,模拟中的 AFGP8 在溶液中优先采用 PPII 螺旋二级结构。我们表明 PPII 螺旋中亲水和疏水基团的分离对于冰结合至关重要。结合是通过肽和二糖的甲基基团吸附到冰上而发生的,这是由疏水基团在它们嵌套在冰表面的空腔中时的脱水熵驱动的。对主棱柱面的选择性源于它与基面相比具有更深的空腔。我们估计了 AFGP8 和更长的 AFGPs4-6 结合的自由能,发现它们与实验中证明的可逆结合一致。模拟结果表明,AFGP8 通过无数构象与冰结合,这些构象用于扩散穿过冰面并找到冰阶,并强烈吸附在冰阶上。我们解释说,多个弱结合位点的存在是 AFGP 异常冰重结晶抑制活性的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号