Phytomedicine ( IF 6.7 ) Pub Date : 2019-02-18 , DOI: 10.1016/j.phymed.2019.152864 Huaying Fan , Zhenfang Gao , Kai Ji , Xin Li , Jingbao Wu , Yue Liu , Xuekai Wang , Haiyue Liang , Yanan Liu , Xiaoting Li , Pan Liu , Daquan Chen , Feng Zhao
|
Background
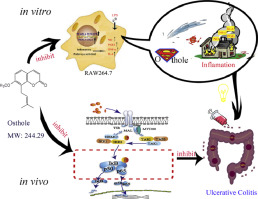
Ulcerative colitis (UC) is a chronic inflammatory condition of the intestines and is difficult to cure once diagnosed. The efficacy of the current clinical treatment for UC is limited. Common anti-inflammatory drugs are prone to adverse effects, while novel biological agents are expensive, although tolerated by patients. Therefore, an urgency exists to find more safe and effective drugs to treat UC. Osthole is an active constituent isolated from the fruit of Cnidium monnieri (L.) Cuss. Osthole has anti-inflammatory activities and offers certain intestinal protection. These characteristics indicate that osthole has the potential to inhibit UC.
Purpose
The study was conducted to investigate the anti-inflammatory potential of osthole in LPS-induced RAW 264.7 cells and dextran sulphate sodium (DSS)-induced ulcerative colitis in mice.
Methods
In in vitro experiments, mouse monocyte-macrophage RAW 264.7 cells were stimulated by 1 μg/ml LPS to produce inflammatory mediators. Griess reagent was used to determine Nitric Oxide (NO) production, and ELISA kits were used to determine the levels of PGE2, TNF-α, and IL-6. The anti-inflammatory mechanisms of osthole were detected using western blot. In in vivo experiments, UC was induced via the intragastric administration of 3.5% DSS to BALB/C mice for 7 days. During the experiment, clinical signs and body weight were monitored and recorded daily to calculate the DAI score. At the end of the experiment, the colon lengths were measured. The colonic histopathological lesions were evaluated. MPO activity and TNF-α levels were determined using the corresponding kits. The protein expression of TNF-α and NF-κB pathways were analysed using western blot.
Results
In an in vitro study, osthole inhibited the production of NO, PGE2, TNF-α, and IL-6 in LPS-induced RAW 264.7 cells. The results of western blot showed that osthole inhibited the expression of iNOS, COX-2, p38 MAPK and IκB α in RAW 264.7 cells. On this basis, in DSS-induced UC mice, it was found that osthole relieved the symptoms of UC by inhibiting weight loss, colon shortening and the DAI score, and simultaneously alleviating colon tissue lesions. It was also found that osthole reduced the levels of TNF-α in serum and colon tissues and effectively inhibited the activity of MPO. The western blot results showed that osthole reduced the expression of NF-κB p65 and p-IκB α and increased the content of IκB α in colon tissues.
Conclusion
Osthole exerted anti-inflammatory effects by blocking the activation of the NF-κB and MAPK/p38 pathways. Additionally, osthole possesses therapeutic potential in the treatment of UC.
中文翻译:

在体外和体内蛇床子素的抗炎作用,从各大天然香豆素蛇床子(L.)CUSS,通过NF-κB和MAPK的激活的阻断/ p38的通路
背景
溃疡性结肠炎(UC)是肠道的一种慢性炎症,一旦确诊就很难治愈。当前用于UC的临床治疗的功效是有限的。普通的抗炎药容易产生不良反应,而新型的生物制剂虽然患者可以耐受,但价格昂贵。因此,迫切需要找到更多安全有效的药物来治疗UC。Osthole是从Cnidium monnieri(L.)Cuss的果实中分离出来的一种活性成分。Osthole具有抗炎活性,并提供一定的肠道保护作用。这些特征表明,osthole具有抑制UC的潜力。
目的
进行这项研究的目的是研究蛇床子素在LPS诱导的RAW 264.7细胞和右旋糖酐硫酸钠(DSS)诱导的小鼠溃疡性结肠炎中的抗炎潜力。
方法
在体外实验中,小鼠单核巨噬细胞RAW 264.7细胞被1μg/ ml LPS刺激以产生炎症介质。使用Griess试剂确定一氧化氮(NO)的产生,并使用ELISA试剂盒确定PGE 2, TNF-α和IL-6的水平。使用蛋白质印迹法检测了蛇床子素的抗炎机制。在体内实验中,通过向BALB / C小鼠胃内施用3.5%DSS诱导7天来诱导UC。在实验过程中,每天监测并记录临床体征和体重,以计算DAI评分。在实验结束时,测量结肠长度。评估结肠的组织病理学病变。使用相应的试剂盒测定MPO活性和TNF-α水平。使用蛋白质印迹分析了TNF-α和NF-κB通路的蛋白表达。
结果
在一项体外研究中,osthole抑制了LPS诱导的RAW 264.7细胞中NO,PGE2,TNF-α和IL-6的产生。蛋白质印迹结果表明,蛇床子素可抑制RAW 264.7细胞中iNOS,COX-2,p38 MAPK和IκBα的表达。在此基础上,在DSS诱导的UC小鼠中,发现osthole通过抑制体重减轻,结肠缩短和DAI评分并同时减轻结肠组织损伤来缓解UC症状。还发现,蛇床子素降低了血清和结肠组织中TNF-α的水平,并有效地抑制了MPO的活性。蛋白质印迹结果显示,蛇床子素可降低结肠组织中NF-κBp65和p-IκBα的表达,并增加IκBα的含量。
结论
osthole通过阻止NF-κB和MAPK / p38途径的激活发挥抗炎作用。另外,osthole在UC的治疗中具有治疗潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号