当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Green, Mild, and Efficient Friedel–Crafts Benzylation of Scarcely Reactive Arenes and Heteroarenes under On‐Water Conditions
ChemSusChem ( IF 7.5 ) Pub Date : 2019-03-19 , DOI: 10.1002/cssc.201900137 Pellegrino La Manna 1 , Annunziata Soriente 1 , Margherita De Rosa 1 , Antonio Buonerba 1 , Carmen Talotta 1 , Carmine Gaeta 1 , Placido Neri 1
ChemSusChem ( IF 7.5 ) Pub Date : 2019-03-19 , DOI: 10.1002/cssc.201900137 Pellegrino La Manna 1 , Annunziata Soriente 1 , Margherita De Rosa 1 , Antonio Buonerba 1 , Carmen Talotta 1 , Carmine Gaeta 1 , Placido Neri 1
Affiliation
|
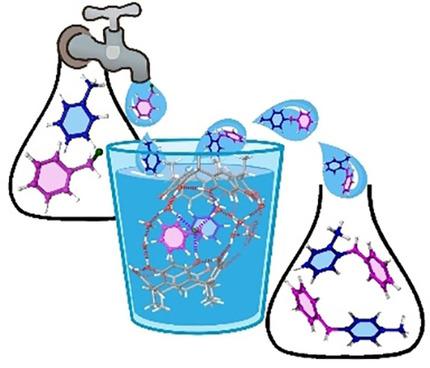
Metal‐free Friedel–Crafts benzylation (FCB) of scarcely reactive arenes and heteroarenes was performed under on‐water conditions by an environmentally sustainable procedure. The catalytic strategy exploits the hydrophobicity of the resorcinarene macrocycle 1 a. The proposed mechanism is based on the activation of benzyl chloride by H‐bonding interactions with catalyst 1 a. In fact, under on‐water conditions the hydrophobic amplification of the strength of the H‐bonding interactions between the OH groups of the resorcinarene catalyst and the chlorine atom of benzyl chloride leads to polarization of the C−Cl bond, which consequently promotes electrophilic attack of the π nucleophile. Thus, many arenes and heteroarenes were efficiently benzylated under mild on‐water conditions by using resorcinarene 1 a as catalyst. The FCB of benzene is industrially relevant for the synthesis of diphenylmethane, and hence the on‐water procedure was extended to the gram‐scale synthesis of diphenylmethane, starting from benzene and benzyl chloride in the presence of resorcinarene catalyst 1 a.
中文翻译:

绿色,温和且高效的弗瑞德尔-弗里德尔工艺在水上条件下几乎不具有活性的芳烃和杂芳烃的苯甲酰化作用
几乎没有反应性的芳烃和杂芳烃的无金属弗里德尔-克拉夫特苄基化反应(FCB)是在水上条件下通过环境可持续的方法进行的。催化策略利用间苯二烯大环1a的疏水性。所提出的机理是基于通过与催化剂1a的H键相互作用来活化苄基氯。实际上,在水上条件下,间苯二甲烯催化剂的OH基团与苄基氯的氯原子之间的H键相互作用强度的疏水性放大导致C-Cl键极化,从而促进了亲电子攻击π亲核试剂。因此,通过使用间苯二甲烯1a作为催化剂,在温和的水条件下,许多芳烃和杂芳烃都被有效地苄基化。苯的FCB在工业上与二苯甲烷的合成有关,因此在间苯二甲烯催化剂1a的存在下,从苯和苄基氯开始,水上程序扩展到克级的二苯甲烷的合成。
更新日期:2019-03-19
中文翻译:

绿色,温和且高效的弗瑞德尔-弗里德尔工艺在水上条件下几乎不具有活性的芳烃和杂芳烃的苯甲酰化作用
几乎没有反应性的芳烃和杂芳烃的无金属弗里德尔-克拉夫特苄基化反应(FCB)是在水上条件下通过环境可持续的方法进行的。催化策略利用间苯二烯大环1a的疏水性。所提出的机理是基于通过与催化剂1a的H键相互作用来活化苄基氯。实际上,在水上条件下,间苯二甲烯催化剂的OH基团与苄基氯的氯原子之间的H键相互作用强度的疏水性放大导致C-Cl键极化,从而促进了亲电子攻击π亲核试剂。因此,通过使用间苯二甲烯1a作为催化剂,在温和的水条件下,许多芳烃和杂芳烃都被有效地苄基化。苯的FCB在工业上与二苯甲烷的合成有关,因此在间苯二甲烯催化剂1a的存在下,从苯和苄基氯开始,水上程序扩展到克级的二苯甲烷的合成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号