当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidines to develop functionalized ligands to target adenosine receptors: fluorescent ligands as an example.
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019-02-18 , DOI: 10.1039/c9md00014c Stephanie Federico 1 , Enrico Margiotta 2 , Silvia Paoletta 2 , Sonja Kachler 3 , Karl-Norbert Klotz 3 , Kenneth A Jacobson 4 , Giorgia Pastorin 5 , Stefano Moro 2 , Giampiero Spalluto 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019-02-18 , DOI: 10.1039/c9md00014c Stephanie Federico 1 , Enrico Margiotta 2 , Silvia Paoletta 2 , Sonja Kachler 3 , Karl-Norbert Klotz 3 , Kenneth A Jacobson 4 , Giorgia Pastorin 5 , Stefano Moro 2 , Giampiero Spalluto 1
Affiliation
|
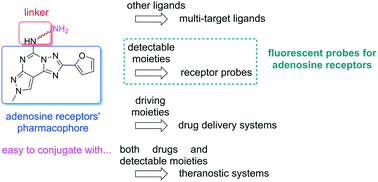
A series of adenosine receptor antagonists bearing a reactive linker was developed. Functionalization of these derivatives is useful to easily obtain multi-target ligands, receptor probes, drug delivery systems, and diagnostic or theranostic systems. The pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine scaffold was chosen as a pharmacophore for the adenosine receptors. It was substituted at the 5 position with reactive linkers of different lengths. Then, these compounds were used to synthesise probes for the adenosine receptors by functionalization with a fluorescent moiety. Both series of compounds were evaluated for their binding at the four adenosine receptor subtypes. Different affinity and selectivity profiles were observed towards hA1, hA2A and hA3 adenosine receptors. In particular, fluorescent compounds behave as dual hA2A/hA3 ligands. Computational studies suggested different binding modes for developed compounds at the three receptors. Both molecular docking and supervised molecular dynamics (SuMD) simulations confirmed that the preferred binding mode at the single receptor was driven by the substitution present at the 5 position. Obtained results rationalized the compounds' binding profile at the adenosine receptors and pave the way for the development of more potent conjugable and conjugated ligands targeting these membrane receptors.
中文翻译:

吡唑并[4,3-e][1,2,4]三唑并[1,5-c]嘧啶开发靶向腺苷受体的功能化配体:以荧光配体为例。
开发了一系列带有反应性接头的腺苷受体拮抗剂。这些衍生物的功能化可用于轻松获得多靶点配体、受体探针、药物递送系统以及诊断或治疗诊断系统。选择吡唑并[4,3-e][1,2,4]三唑并[1,5-c]嘧啶支架作为腺苷受体的药效基团。它在 5 位被不同长度的反应性接头取代。然后,这些化合物通过荧光部分的功能化来合成腺苷受体的探针。评估了这两个系列的化合物与四种腺苷受体亚型的结合。观察到对 hA1、hA2A 和 hA3 腺苷受体的不同亲和力和选择性特征。特别是,荧光化合物充当双 hA2A/hA3 配体。计算研究表明已开发的化合物在三种受体上具有不同的结合模式。分子对接和监督分子动力学 (SuMD) 模拟均证实,单个受体的首选结合模式是由 5 位上的取代驱动的。获得的结果合理化了化合物在腺苷受体上的结合特征,并为开发针对这些膜受体的更有效的可缀合和缀合配体铺平了道路。
更新日期:2019-02-18
中文翻译:

吡唑并[4,3-e][1,2,4]三唑并[1,5-c]嘧啶开发靶向腺苷受体的功能化配体:以荧光配体为例。
开发了一系列带有反应性接头的腺苷受体拮抗剂。这些衍生物的功能化可用于轻松获得多靶点配体、受体探针、药物递送系统以及诊断或治疗诊断系统。选择吡唑并[4,3-e][1,2,4]三唑并[1,5-c]嘧啶支架作为腺苷受体的药效基团。它在 5 位被不同长度的反应性接头取代。然后,这些化合物通过荧光部分的功能化来合成腺苷受体的探针。评估了这两个系列的化合物与四种腺苷受体亚型的结合。观察到对 hA1、hA2A 和 hA3 腺苷受体的不同亲和力和选择性特征。特别是,荧光化合物充当双 hA2A/hA3 配体。计算研究表明已开发的化合物在三种受体上具有不同的结合模式。分子对接和监督分子动力学 (SuMD) 模拟均证实,单个受体的首选结合模式是由 5 位上的取代驱动的。获得的结果合理化了化合物在腺苷受体上的结合特征,并为开发针对这些膜受体的更有效的可缀合和缀合配体铺平了道路。































 京公网安备 11010802027423号
京公网安备 11010802027423号