当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-02-18 , DOI: 10.1038/s41419-019-1425-4
Tingting Yu 1 , Xin Zhang 1, 2 , Haosong Shi 3 , Jinge Tian 1, 4 , Lingling Sun 1 , Xueming Hu 5 , Wenqiang Cui 5 , Dongping Du 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-02-18 , DOI: 10.1038/s41419-019-1425-4
Tingting Yu 1 , Xin Zhang 1, 2 , Haosong Shi 3 , Jinge Tian 1, 4 , Lingling Sun 1 , Xueming Hu 5 , Wenqiang Cui 5 , Dongping Du 1
Affiliation
|
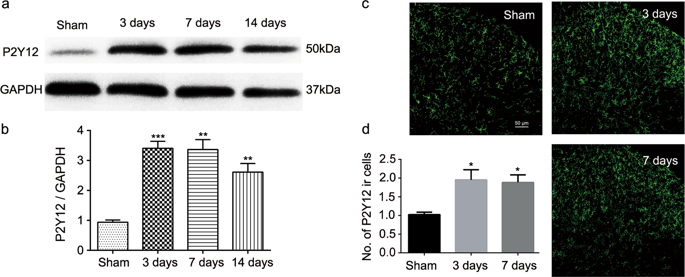
Peripheral nerve injury causes neuropathic pain and microglia activation. P2Y12 receptors on microglia are thought to be a key player in the surveillance of the local environment, but whether or how these receptors are engaged in the cross-talk between microglia and neurons of the dorsal horn remain ambiguous. Using a rodent model of nerve injury-induced pain, we investigated the roles of P2Y12 in microglia activation, excitatory synaptic transmission, and nociceptive allodynia. We found that spinal nerve ligation (SNL) significantly increased the level of P2Y12 receptors specifically in the microglia of the ipsilateral dorsal horn. Injections of P2Y12 antagonists (MRS2395 or clopidogrel) attenuated microglia activation and increased the paw withdrawal latency in response to thermal stimuli on the ipsilateral side without affecting the basal threshold on the contralateral side. These effects on pain behaviors were replicated in P2Y12 knockout mice. Patch-clamp recordings further revealed that partial sciatic nerve ligation (PSNL)-induced excessive miniature excitatory postsynaptic currents (mEPSCs) were significantly attenuated in P2Y12 knockout mice. Moreover, we found that SNL activates the GTP-RhoA/ROCK2 signaling pathway and elevates the level of phosphorylated p38 mitogen-activated protein kinase (MAPK), which was inhibited by the P2Y12 antagonist. The phosphorylation of p38 MAPK was inhibited by a ROCK inhibitor, but not vice versa, suggesting that p38 MAPK is downstream of ROCK activation. Our findings suggest that nerve injury engages the P2Y12 receptor-dependent GTP-RhoA/ROCK2 signaling pathway to upregulate excitatory synaptic transmission in the dorsal horn. This cross-talk ultimately participates in the manifestation of nociceptive allodynia, implicating P2Y12 receptor as a potential target for alleviating neuropathic pain.
中文翻译:

P2Y12调节啮齿类动物神经性疼痛过程中脊髓板层II神经元的小胶质细胞激活和兴奋性突触传递。
周围神经损伤导致神经性疼痛和小胶质细胞活化。小胶质细胞上的P2Y12受体被认为是监测局部环境的关键因素,但是这些受体是否参与小胶质细胞和背角神经元之间的串扰仍是模棱两可的。使用神经损伤引起的疼痛的啮齿动物模型,我们调查了P2Y12在小胶质细胞激活,兴奋性突触传递和伤害性异常性疼痛中的作用。我们发现,脊髓神经结扎(SNL)显着增加了P2Y12受体的水平,特别是在同侧背角的小胶质细胞中。P2Y12拮抗剂(MRS2395或氯吡格雷)的注射减弱了小胶质细胞的活化并增加了对同侧热刺激的爪缩回潜伏期,而不会影响对侧的基础阈值。这些对疼痛行为的影响已在P2Y12基因敲除小鼠中复制。膜片钳记录进一步揭示,在P2Y12基因敲除小鼠中,部分坐骨神经结扎(PSNL)诱导的过度微型兴奋性突触后电流(mEPSCs)明显减弱。此外,我们发现SNL激活GTP-RhoA / ROCK2信号传导途径,并提高了P2Y12拮抗剂抑制的磷酸化p38丝裂原活化蛋白激酶(MAPK)的水平。ROCK抑制剂可抑制p38 MAPK的磷酸化,反之亦然,提示p38 MAPK在ROCK激活的下游。我们的发现表明神经损伤参与了P2Y12受体依赖性GTP-RhoA / ROCK2信号通路,从而上调了背角的兴奋性突触传递。这种串扰最终参与了伤害性异常性疼痛的表现,暗示P2Y12受体可能是减轻神经性疼痛的潜在靶标。
更新日期:2019-02-19
中文翻译:

P2Y12调节啮齿类动物神经性疼痛过程中脊髓板层II神经元的小胶质细胞激活和兴奋性突触传递。
周围神经损伤导致神经性疼痛和小胶质细胞活化。小胶质细胞上的P2Y12受体被认为是监测局部环境的关键因素,但是这些受体是否参与小胶质细胞和背角神经元之间的串扰仍是模棱两可的。使用神经损伤引起的疼痛的啮齿动物模型,我们调查了P2Y12在小胶质细胞激活,兴奋性突触传递和伤害性异常性疼痛中的作用。我们发现,脊髓神经结扎(SNL)显着增加了P2Y12受体的水平,特别是在同侧背角的小胶质细胞中。P2Y12拮抗剂(MRS2395或氯吡格雷)的注射减弱了小胶质细胞的活化并增加了对同侧热刺激的爪缩回潜伏期,而不会影响对侧的基础阈值。这些对疼痛行为的影响已在P2Y12基因敲除小鼠中复制。膜片钳记录进一步揭示,在P2Y12基因敲除小鼠中,部分坐骨神经结扎(PSNL)诱导的过度微型兴奋性突触后电流(mEPSCs)明显减弱。此外,我们发现SNL激活GTP-RhoA / ROCK2信号传导途径,并提高了P2Y12拮抗剂抑制的磷酸化p38丝裂原活化蛋白激酶(MAPK)的水平。ROCK抑制剂可抑制p38 MAPK的磷酸化,反之亦然,提示p38 MAPK在ROCK激活的下游。我们的发现表明神经损伤参与了P2Y12受体依赖性GTP-RhoA / ROCK2信号通路,从而上调了背角的兴奋性突触传递。这种串扰最终参与了伤害性异常性疼痛的表现,暗示P2Y12受体可能是减轻神经性疼痛的潜在靶标。































 京公网安备 11010802027423号
京公网安备 11010802027423号