Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Studies on the Nonenzymatic Deamidation Mechanisms of Glutamine Residues
ACS Omega ( IF 3.7 ) Pub Date : 2019-02-18 00:00:00 , DOI: 10.1021/acsomega.8b03199 Koichi Kato 1, 2 , Tomoki Nakayoshi 2 , Eiji Kurimoto 2 , Akifumi Oda 2, 3
ACS Omega ( IF 3.7 ) Pub Date : 2019-02-18 00:00:00 , DOI: 10.1021/acsomega.8b03199 Koichi Kato 1, 2 , Tomoki Nakayoshi 2 , Eiji Kurimoto 2 , Akifumi Oda 2, 3
Affiliation
|
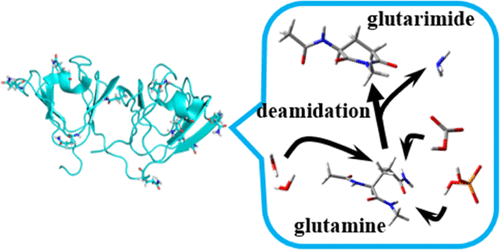
The nonenzymatic deamidation reactions of asparagine (Asn) and glutamine (Gln) residues in proteins are associated with protein turnover and age-related diseases. The reactions are also believed to provide a molecular clock for biological processes. Although Gln deamidation is assumed to occur through the glutarimide intermediate, the mechanisms for this are unclear because under normal physiological conditions, Gln deamidation occurs relatively less frequently and at a lower rate than Asn deamidation. We investigate the mechanisms underlying glutarimide formation from Gln residues, which proceeds in two steps (cyclization and deammoniation) catalyzed by phosphate and carbonate. We also compare these reactions with noncatalytic mechanisms and water-catalyzed mechanisms. The calculations were performed on the model compound Ace–Gln–Nme (Ace = acetyl, Nme = methylamino) using the density functional theory with the B3LYP/6-31+G(d,p) level of theory. Our results suggest that all the catalysts used in our study can mediate the proton relays required for glutarimide formation. We further determined that the calculated activation barriers of the reactions catalyzed by phosphate ions (115 kJ mol–1) and carbonate ions (112 kJ mol–1) are sufficiently low for the reactions to occur under normal physiological conditions. We also show that nucleophilic enhancement of Nme nitrogen is essential for the cyclization of Gln residues.
中文翻译:

谷氨酰胺残基非酶脱酰胺机理的计算研究
蛋白质中天冬酰胺(Asn)和谷氨酰胺(Gln)残基的非酶脱酰胺反应与蛋白质更新和与年龄有关的疾病有关。还认为该反应为生物过程提供了分子钟。尽管假定Gln脱酰胺作用是通过戊二酰亚胺中间体发生的,但其机制尚不清楚,因为在正常的生理条件下,Gln脱酰胺作用的发生频率相对较低,且发生率低于Asn脱酰胺作用。我们研究了由Gln残基形成戊二酰亚胺的机理,该机理分两步进行(环化和脱氨),分别由磷酸盐和碳酸盐催化。我们还将这些反应与非催化机理和水催化机理进行了比较。使用密度泛函理论和B3LYP / 6-31 + G(d,p)的理论水平,对模型化合物Ace–Gln–Nme(Ace =乙酰基,Nme =甲基氨基)进行了计算。我们的结果表明,在我们的研究中使用的所有催化剂都可以介导戊二酰亚胺形成所需的质子传递。我们进一步确定,计算得出的由磷酸根离子(115 kJ mol–1)和碳酸根离子(112 kJ mol –1)足够低,足以使反应在正常生理条件下发生。我们还表明,Nme氮的亲核性增强对于Gln残基的环化必不可少。
更新日期:2019-02-18
中文翻译:

谷氨酰胺残基非酶脱酰胺机理的计算研究
蛋白质中天冬酰胺(Asn)和谷氨酰胺(Gln)残基的非酶脱酰胺反应与蛋白质更新和与年龄有关的疾病有关。还认为该反应为生物过程提供了分子钟。尽管假定Gln脱酰胺作用是通过戊二酰亚胺中间体发生的,但其机制尚不清楚,因为在正常的生理条件下,Gln脱酰胺作用的发生频率相对较低,且发生率低于Asn脱酰胺作用。我们研究了由Gln残基形成戊二酰亚胺的机理,该机理分两步进行(环化和脱氨),分别由磷酸盐和碳酸盐催化。我们还将这些反应与非催化机理和水催化机理进行了比较。使用密度泛函理论和B3LYP / 6-31 + G(d,p)的理论水平,对模型化合物Ace–Gln–Nme(Ace =乙酰基,Nme =甲基氨基)进行了计算。我们的结果表明,在我们的研究中使用的所有催化剂都可以介导戊二酰亚胺形成所需的质子传递。我们进一步确定,计算得出的由磷酸根离子(115 kJ mol–1)和碳酸根离子(112 kJ mol –1)足够低,足以使反应在正常生理条件下发生。我们还表明,Nme氮的亲核性增强对于Gln残基的环化必不可少。






























 京公网安备 11010802027423号
京公网安备 11010802027423号