当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ketenimine Formation Catalyzed by a High-Valent Cobalt Carbene in Bulky Alkoxide Ligand Environment
Organometallics ( IF 2.5 ) Pub Date : 2019-02-15 , DOI: 10.1021/acs.organomet.8b00911 Amanda Grass 1 , Nicholas S. Dewey 2 , Richard L. Lord 2 , Stanislav Groysman 1
Organometallics ( IF 2.5 ) Pub Date : 2019-02-15 , DOI: 10.1021/acs.organomet.8b00911 Amanda Grass 1 , Nicholas S. Dewey 2 , Richard L. Lord 2 , Stanislav Groysman 1
Affiliation
|
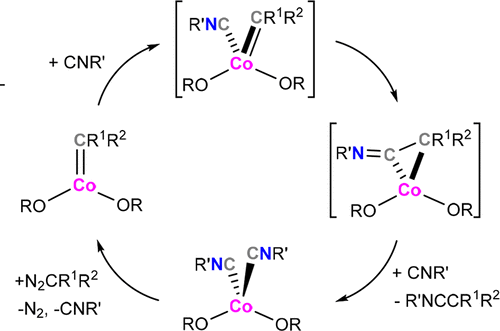
High-valent cobalt carbene Co(OR)2(═CPh2) (OR = OCtBu2Ph) undergoes reaction with various isocyanides CNR′ (CNR′ = 2,6-dimethylphenyl isocyanide, 4-methoxyphenyl isocyanide, 2-chloro-6-methylphenyl isocyanide, adamantyl isocyanide) to yield the corresponding ketenimine. The reaction is accompanied by the formation of cobalt bis(alkoxide) bis(isocyanide) complexes Co(OR)2(CNR)2, which were independently synthesized and characterized. DFT calculations suggest the mechanism proceeds through isocyanide binding to Co, followed by intramolecular insertion into the Co–carbene bond to form the ketenimine. We have also conducted an investigation of the catalytic formation of ketenimines at room temperature using mixtures of diazoalkanes (diphenyldiazomethane, methyl diazo(phenyl)acetate, and ethyl diazoacetate) and isocyanides (2,6-dimethylphenyl isocyanide, 4-methoxyphenyl isocyanide, adamantyl isocyanide, cyclohexyl isocyanide, and benzyl isocyanide). While no catalytic reactivity was observed for diphenyldiazomethane, ester-substituted diazoalkanes (diazoesters) demonstrate catalytic turnover. Relatively high yields are observed for the reactions involving bulkier aliphatic substrates adamantyl and cyclohexyl isocyanides. Mechanistic studies suggest that the lack of catalytic reactivity involving diphenyldiazomethane results from the inability of Co(OR)2(CNR)2 to undergo carbene formation upon reaction with N2CPh2. In contrast, facile reaction is observed between Co(OR)2(CNR)2 and diazoesters, which enables the overall catalytic reactivity.
中文翻译:

大体积醇盐配体环境中高价钴碳催化酮丁胺的形成
高价钴卡宾的Co(OR)2(═CPh 2)(OR = OC吨卜2 PH)经历与各种异氰化物CNR'(CNR'= 2,6-二甲基苯基胩,4-甲氧基苯基异腈,2-氯反应-6-甲基苯基异氰化物,金刚烷基异氰化物)得到相应的酮亚胺。该反应伴随有钴双(醇盐)双(异氰酸酯)配合物Co(OR)2(CNR)2的形成,它们是独立合成和表征的。DFT计算表明,机理是通过异氰酸酯与Co结合,然后分子内插入Co-卡宾键形成酮亚胺而进行的。我们还使用重氮烷烃(二苯基重氮甲烷,甲基重氮(苯基)乙酸盐和重氮乙酸乙酯)和异氰酸酯(2,6-二甲基苯基异氰酸酯,4-甲氧基苯基异氰酸酯,金刚烷基异氰酸酯)的混合物在室温下催化了酮亚胺的形成。 ,环己基异氰化物和苄基异氰化物)。虽然未观察到二苯基重氮甲烷的催化反应性,但酯取代的重氮烷烃(重氮酯)显示出催化转化率。对于涉及较大的脂族底物金刚烷基和环己基异氰化物的反应,观察到相对较高的产率。2(CNR)2在与N 2 CPh 2反应时经历卡宾形成。相反,在Co(OR)2(CNR)2和重氮酯之间观察到容易的反应,这使得总催化反应性成为可能。
更新日期:2019-02-19
中文翻译:

大体积醇盐配体环境中高价钴碳催化酮丁胺的形成
高价钴卡宾的Co(OR)2(═CPh 2)(OR = OC吨卜2 PH)经历与各种异氰化物CNR'(CNR'= 2,6-二甲基苯基胩,4-甲氧基苯基异腈,2-氯反应-6-甲基苯基异氰化物,金刚烷基异氰化物)得到相应的酮亚胺。该反应伴随有钴双(醇盐)双(异氰酸酯)配合物Co(OR)2(CNR)2的形成,它们是独立合成和表征的。DFT计算表明,机理是通过异氰酸酯与Co结合,然后分子内插入Co-卡宾键形成酮亚胺而进行的。我们还使用重氮烷烃(二苯基重氮甲烷,甲基重氮(苯基)乙酸盐和重氮乙酸乙酯)和异氰酸酯(2,6-二甲基苯基异氰酸酯,4-甲氧基苯基异氰酸酯,金刚烷基异氰酸酯)的混合物在室温下催化了酮亚胺的形成。 ,环己基异氰化物和苄基异氰化物)。虽然未观察到二苯基重氮甲烷的催化反应性,但酯取代的重氮烷烃(重氮酯)显示出催化转化率。对于涉及较大的脂族底物金刚烷基和环己基异氰化物的反应,观察到相对较高的产率。2(CNR)2在与N 2 CPh 2反应时经历卡宾形成。相反,在Co(OR)2(CNR)2和重氮酯之间观察到容易的反应,这使得总催化反应性成为可能。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号