当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ordered and Isomerically Stable Bicyclic Peptide Scaffolds Constrained through Cystine Bridges and Proline Turns.
ChemBioChem ( IF 2.6 ) Pub Date : 2019-04-18 , DOI: 10.1002/cbic.201800788 Ping Lin 1 , Hongwei Yao 1 , Jun Zha 1 , Yibing Zhao 1 , Chuanliu Wu 1
ChemBioChem ( IF 2.6 ) Pub Date : 2019-04-18 , DOI: 10.1002/cbic.201800788 Ping Lin 1 , Hongwei Yao 1 , Jun Zha 1 , Yibing Zhao 1 , Chuanliu Wu 1
Affiliation
|
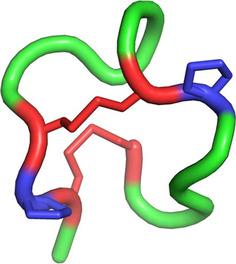
Bicyclic peptides are attractive scaffolds for the design of potent protein binders and new therapeutics. However, peptide bicycles constrained through disulfide bonds are rarely stable or tolerant to sequence manipulation owing to disulfide isomerization, especially for peptides lacking a regular secondary structure. Herein, we report the discovery and identification of a class of bicyclic peptide scaffolds with ordered but irregular secondary structures. These peptides have a conserved cysteine/proline framework for directing the oxidative folding into a fused bicyclic structure that consists of four irregular turns and a 310 helix (characterized by NMR spectroscopy). This work shows that bicyclic peptides can be stabilized into ordered structures by manipulating both the disulfide bonds and proline-stabilized turns. In turn, this could inspire the design and engineering of multicyclic peptides with new structures and benefit the development of novel protein binders and therapeutics.
中文翻译:

通过胱氨酸桥和脯氨酸转弯限制的有序和异构稳定的双环肽支架。
双环肽是用于设计有效的蛋白质结合剂和新疗法的有吸引力的支架。然而,由于二硫键异构化,受二硫键约束的肽自行车很少稳定或不能耐受序列操纵,尤其是对于缺乏规则二级结构的肽。在这里,我们报告发现和鉴定一类有序但不规则的二级结构的双环肽支架。这些肽具有保守的半胱氨酸/脯氨酸框架,用于指导氧化折叠成稠密的双环结构,该结构由四个不规则匝和310螺旋组成(通过NMR光谱表征)。这项工作表明,双环肽可以通过操纵二硫键和脯氨酸稳定的匝数来稳定成有序结构。反过来,
更新日期:2019-04-18
中文翻译:

通过胱氨酸桥和脯氨酸转弯限制的有序和异构稳定的双环肽支架。
双环肽是用于设计有效的蛋白质结合剂和新疗法的有吸引力的支架。然而,由于二硫键异构化,受二硫键约束的肽自行车很少稳定或不能耐受序列操纵,尤其是对于缺乏规则二级结构的肽。在这里,我们报告发现和鉴定一类有序但不规则的二级结构的双环肽支架。这些肽具有保守的半胱氨酸/脯氨酸框架,用于指导氧化折叠成稠密的双环结构,该结构由四个不规则匝和310螺旋组成(通过NMR光谱表征)。这项工作表明,双环肽可以通过操纵二硫键和脯氨酸稳定的匝数来稳定成有序结构。反过来,































 京公网安备 11010802027423号
京公网安备 11010802027423号