Nature Immunology ( IF 27.7 ) Pub Date : 2019-02-18 , DOI: 10.1038/s41590-019-0312-6 Brian C. Miller , Debattama R. Sen , Rose Al Abosy , Kevin Bi , Yamini V. Virkud , Martin W. LaFleur , Kathleen B. Yates , Ana Lako , Kristen Felt , Girish S. Naik , Michael Manos , Evisa Gjini , Juhi R. Kuchroo , Jeffrey J. Ishizuka , Jenna L. Collier , Gabriel K. Griffin , Seth Maleri , Dawn E. Comstock , Sarah A. Weiss , Flavian D. Brown , Arpit Panda , Margaret D. Zimmer , Robert T. Manguso , F. Stephen Hodi , Scott J. Rodig , Arlene H. Sharpe , W. Nicholas Haining
|
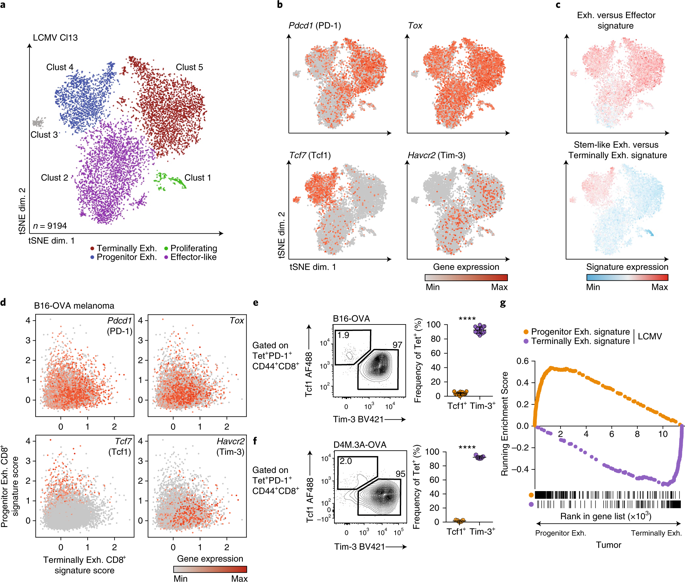
T cell dysfunction is a hallmark of many cancers, but the basis for T cell dysfunction and the mechanisms by which antibody blockade of the inhibitory receptor PD-1 (anti-PD-1) reinvigorates T cells are not fully understood. Here we show that such therapy acts on a specific subpopulation of exhausted CD8+ tumor-infiltrating lymphocytes (TILs). Dysfunctional CD8+ TILs possess canonical epigenetic and transcriptional features of exhaustion that mirror those seen in chronic viral infection. Exhausted CD8+ TILs include a subpopulation of ‘progenitor exhausted’ cells that retain polyfunctionality, persist long term and differentiate into ‘terminally exhausted’ TILs. Consequently, progenitor exhausted CD8+ TILs are better able to control tumor growth than are terminally exhausted T cells. Progenitor exhausted TILs can respond to anti-PD-1 therapy, but terminally exhausted TILs cannot. Patients with melanoma who have a higher percentage of progenitor exhausted cells experience a longer duration of response to checkpoint-blockade therapy. Thus, approaches to expand the population of progenitor exhausted CD8+ T cells might be an important component of improving the response to checkpoint blockade.
中文翻译:

精疲力竭的CD8 + T细胞亚群介导肿瘤控制并响应检查站封锁
T细胞功能障碍是许多癌症的标志,但是T细胞功能障碍的基础以及抑制受体PD-1(抗PD-1)的抗体重新激活T细胞的机制尚未完全清楚。在这里,我们表明,这种疗法作用于精疲力竭的CD8 +肿瘤浸润淋巴细胞(TILs)的特定亚群。功能失调的CD8 + TIL具有典型的疲劳衰竭表观遗传和转录特征,与慢性病毒感染中的特征相似。精疲力竭的CD8 + TILs包括“祖细胞精疲力竭”的细胞亚群,这些细胞保留了多功能性,可以长期持续并分化为“终末精疲力竭”的TILs。结果,祖细胞耗尽了CD8 +TIL比最终耗尽的T细胞更能控制肿瘤的生长。祖先精疲力竭的TIL可以对抗PD-1治疗产生反应,但终极精疲力竭的TIL则不能。黑色素瘤患者的祖细胞耗竭细胞百分比更高,对检查点封锁疗法的反应时间更长。因此,扩大祖细胞耗竭的CD8 + T细胞群体的方法可能是改善对检查站封锁反应的重要组成部分。































 京公网安备 11010802027423号
京公网安备 11010802027423号