当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclohexyl Bromide and Iodide: Direct Reduction at Vitreous Carbon Cathodes together with Nickel(I) Salen‐ and Cobalt(I) Salen‐Catalyzed Reductions in Dimethylformamide
ChemElectroChem ( IF 3.5 ) Pub Date : 2017-08-24 , DOI: 10.1002/celc.201700664 Benjamin H. R. Gerroll 1 , Sean P. Bird 1 , Erin T. Martin 1 , Mohammad S. Mubarak 2 , Dennis G. Peters 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2017-08-24 , DOI: 10.1002/celc.201700664 Benjamin H. R. Gerroll 1 , Sean P. Bird 1 , Erin T. Martin 1 , Mohammad S. Mubarak 2 , Dennis G. Peters 1
Affiliation
|
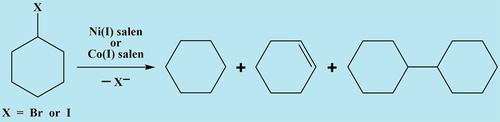
Cyclic voltammetry and controlled‐potential (bulk) electrolysis have been used to study the direct electrochemical reduction of cyclohexyl bromide (1) and cyclohexyl iodide (2) at glassy carbon cathodes in dimethylformamide (DMF) containing 0.10 M tetramethylammonium tetrafluoroborate (TMABF4). Direct reduction of 1 is a one‐step process that affords a carbanion intermediate, whereas 2 undergoes stepwise reduction to a radical and then a carbanion intermediate. Mixtures of cyclohexane, cyclohexene, and bicyclohexyl arise from bulk electrolyses of both 1 and 2. Catalytic reduction of 1 and 2 by nickel(I) salen and cobalt(I) salen electrogenerated at glassy carbon cathodes in DMF‐TMABF4 has been investigated with the aid of both cyclic voltammetry and bulk electrolysis. Products arising from these catalytic reductions are cyclohexane, cyclohexene, and bicyclohexyl, although significant amounts of unreduced 1 are found when cobalt(I) salen is utilized as the catalyst. Mechanistic aspects of the direct and catalyzed reductions of 1 and 2 are discussed.
中文翻译:

环己基溴化物和碘化物:在玻璃碳阴极上直接还原,以及镍(I)Salen-和钴(I)Salen-催化的二甲基甲酰胺还原
循环伏安法和可控电位(本体)电解已用于研究在含0.10 M四氟硼酸四甲基铵(TMABF 4)的二甲基甲酰胺(DMF)中玻璃碳阴极上直接还原环己基溴化物(1)和环己基碘化物(2)的方法。直接还原1是提供碳负离子中间体的一步过程,而2则经历逐步还原为自由基然后是碳负离子中间体的过程。环己烷,环己烯和双环己基的混合物由1和2的本体电解产生。1和2的催化还原在循环伏安法和本体电解法的帮助下,已经研究了在DMF-TMABF 4中在玻碳阴极上电生成的镍(I)Salen和钴(I)Salen的方法。这些催化还原反应产生的产物是环己烷,环己烯和双环己基,尽管当使用钴(I)salen作为催化剂时会发现大量未还原的1。讨论了直接还原和催化还原1和2的机理。
更新日期:2017-08-24
中文翻译:

环己基溴化物和碘化物:在玻璃碳阴极上直接还原,以及镍(I)Salen-和钴(I)Salen-催化的二甲基甲酰胺还原
循环伏安法和可控电位(本体)电解已用于研究在含0.10 M四氟硼酸四甲基铵(TMABF 4)的二甲基甲酰胺(DMF)中玻璃碳阴极上直接还原环己基溴化物(1)和环己基碘化物(2)的方法。直接还原1是提供碳负离子中间体的一步过程,而2则经历逐步还原为自由基然后是碳负离子中间体的过程。环己烷,环己烯和双环己基的混合物由1和2的本体电解产生。1和2的催化还原在循环伏安法和本体电解法的帮助下,已经研究了在DMF-TMABF 4中在玻碳阴极上电生成的镍(I)Salen和钴(I)Salen的方法。这些催化还原反应产生的产物是环己烷,环己烯和双环己基,尽管当使用钴(I)salen作为催化剂时会发现大量未还原的1。讨论了直接还原和催化还原1和2的机理。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号