当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Vapor–Liquid Equilibria and Thermodynamic Modeling of the Methanol + n-Heptane and 1-Butanol + Aniline Binary Systems
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acs.jced.7b00766 Mehran Alinejhad 1 , Alireza Shariati 1 , Javad Hekayati 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acs.jced.7b00766 Mehran Alinejhad 1 , Alireza Shariati 1 , Javad Hekayati 1
Affiliation
|
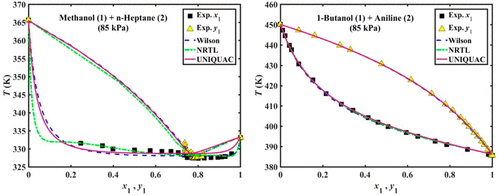
Isobaric vapor–liquid equilibria (VLE) of the binary systems of methanol + n-heptane and 1-butanol + aniline were measured at 85 kPa. The experiments were carried out using an Othmer still with the objective of measuring the equilibrium temperature, as well as the composition of the vapor and liquid phases as determined by gas chromatography analysis. According to the experimental results obtained, both of the binary mixtures studied demonstrate positive deviation from ideal behavior. Furthermore, a point of minimum-boiling azeotrope was observed for the methanol + n-heptane system. The VLE data measured have been verified to be thermodynamically consistent based on the Wisniak modification of the Herington area test for isobaric VLE data. Additionally, the two systems were thermodynamically modeled. Because of the relatively low pressure involved in this work, the modified Raoult’s law was utilized for this purpose. In this regard, the experimental vapor–liquid equilibrium data were correlated with different excess Gibbs energy models, including the Wilson, the nonrandom two-liquid (NRTL), and the universal quasi-chemical (UNIQUAC) activity coefficient models. The results obtained show reasonably good agreement with the experimental data.
中文翻译:

甲醇+正庚烷和1-丁醇+苯胺二元系统的实验汽液平衡和热力学模型
甲醇+正庚烷和1-丁醇+苯胺的二元系统的等压气液平衡(VLE)在85 kPa时测量。使用Othmer蒸馏器进行实验,其目的是测量平衡温度以及通过气相色谱分析确定的气相和液相的组成。根据获得的实验结果,研究的两种二元混合物均显示出与理想行为的正偏差。此外,观察到甲醇+ n的最低沸点共沸点-庚烷系统。基于对等压VLE数据的Herington面积测试的Wisniak修改,已验证所测得的VLE数据在热力学上是一致的。另外,对两个系统进行了热力学建模。由于这项工作涉及的压力相对较低,为此目的采用了修改后的拉乌尔定律。在这方面,实验性的气液平衡数据与不同的过剩吉布斯能量模型相关,包括威尔逊模型,非随机二液体(NRTL)模型和通用拟化学(UNIQUAC)活度系数模型。获得的结果显示与实验数据相当良好的一致性。
更新日期:2018-01-31
中文翻译:

甲醇+正庚烷和1-丁醇+苯胺二元系统的实验汽液平衡和热力学模型
甲醇+正庚烷和1-丁醇+苯胺的二元系统的等压气液平衡(VLE)在85 kPa时测量。使用Othmer蒸馏器进行实验,其目的是测量平衡温度以及通过气相色谱分析确定的气相和液相的组成。根据获得的实验结果,研究的两种二元混合物均显示出与理想行为的正偏差。此外,观察到甲醇+ n的最低沸点共沸点-庚烷系统。基于对等压VLE数据的Herington面积测试的Wisniak修改,已验证所测得的VLE数据在热力学上是一致的。另外,对两个系统进行了热力学建模。由于这项工作涉及的压力相对较低,为此目的采用了修改后的拉乌尔定律。在这方面,实验性的气液平衡数据与不同的过剩吉布斯能量模型相关,包括威尔逊模型,非随机二液体(NRTL)模型和通用拟化学(UNIQUAC)活度系数模型。获得的结果显示与实验数据相当良好的一致性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号