Molecular Cell ( IF 14.5 ) Pub Date : 2018-01-30 , DOI: 10.1016/j.molcel.2017.12.024
Lixin Wan,Kexin Xu,Yongkun Wei,Jinfang Zhang,Tao Han,Christopher Fry,Zhao Zhang,Yao Vickie Wang,Liyu Huang,Min Yuan,Weiya Xia,Wei-Chao Chang,Wen-Chien Huang,Chien-Liang Liu,Yuan-Ching Chang,Jinsong Liu,Yun Wu,Victor X Jin,Xiangpeng Dai,Jianfeng Guo,Jia Liu,Shulong Jiang,Jin Li,John M Asara,Myles Brown,Mien-Chie Hung,Wenyi Wei
|
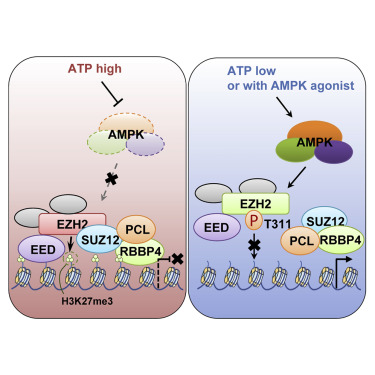
Sustained energy starvation leads to activation of AMP-activated protein kinase (AMPK), which coordinates energy status with numerous cellular processes including metabolism, protein synthesis, and autophagy. Here, we report that AMPK phosphorylates the histone methyltransferase EZH2 at T311 to disrupt the interaction between EZH2 and SUZ12, another core component of the polycomb repressive complex 2 (PRC2), leading to attenuated PRC2-dependent methylation of histone H3 at Lys27. As such, PRC2 target genes, many of which are known tumor suppressors, were upregulated upon T311-EZH2 phosphorylation, which suppressed tumor cell growth both in cell culture and mouse xenografts. Pathologically, immunohistochemical analyses uncovered a positive correlation between AMPK activity and pT311-EZH2, and higher pT311-EZH2 correlates with better survival in both ovarian and breast cancer patients. Our finding suggests that AMPK agonists might be promising sensitizers for EZH2-targeting cancer therapies.
中文翻译:

AMPK对EZH2的磷酸化抑制PRC2甲基转移酶活性和致癌功能。
持续的能量匮乏会导致AMP激活的蛋白激酶(AMPK)激活,从而与许多细胞过程(包括代谢,蛋白质合成和自噬)协调能量状态。在这里,我们报道AMPK在T311处磷酸化组蛋白甲基转移酶EZH2,破坏EZH2和SUZ12之间的相互作用,SUZ12是多梳抑制复合物2(PRC2)的另一个核心成分,导致在Lys27处组蛋白H3的PRC2依赖性甲基化减弱。因此,PRC2靶基因(其中许多是已知的肿瘤抑制因子)在T311-EZH2磷酸化后被上调,从而抑制了肿瘤细胞在细胞培养和小鼠异种移植物中的生长。在病理学上,免疫组化分析发现AMPK活性与pT311-EZH2之间呈正相关,较高的pT311-EZH2与卵巢癌和乳腺癌患者的更好生存相关。我们的发现表明,AMPK激动剂可能是针对EZH2靶向癌症治疗的有希望的敏化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号