当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical Investigation of the Reactivity of Sodium Dicyanamide with Nitric Acid
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.jpca.7b11661 Kristen M. Vogelhuber 1, 2 , Ryan S. Booth 1, 2 , Christopher J. Annesley 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.jpca.7b11661 Kristen M. Vogelhuber 1, 2 , Ryan S. Booth 1, 2 , Christopher J. Annesley 1
Affiliation
|
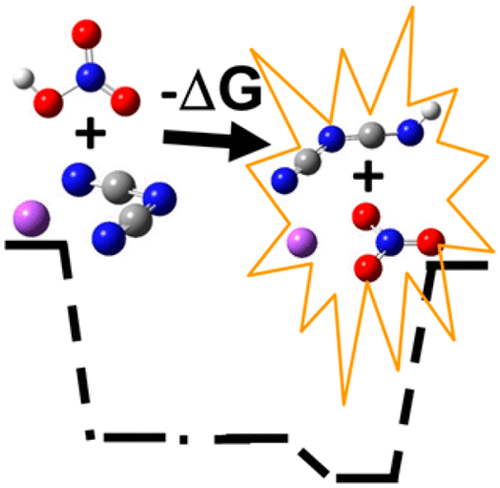
There is a need to replace current hydrazine fuels with safer propellants, and dicyanamide (DCA–)-based systems have emerged as promising alternatives because they autoignite when mixed with some oxidizers. Previous studies of the hypergolic reaction mechanism have focused on the reaction between DCA– and the oxidizer HNO3; here, we compare the calculated pathway of DCA– + HNO3 with the reaction coordinate of the ion pair sodium dicyanamide with nitric acid, Na[DCA] + HNO3. Enthalpies and free energies are calculated in the gas phase and in solution using a quantum mechanical continuum solvation model, SMD-GIL. The barriers to the Na[DCA] + HNO3 reaction are dramatically lowered relative to those of the reaction with the bare anion, and an exothermic exit channel to produce NaNO3 and the reactive intermediate HDCA appears. These results suggest that Na[DCA] may accelerate the ignition reaction.
中文翻译:

双氰胺钠与硝酸反应性的理论研究
有必要用更安全的推进剂代替目前的肼燃料,基于双氰胺(DCA –)的系统已成为有希望的替代品,因为它们与某些氧化剂混合时会自动着火。以前对高声反应机理的研究集中在DCA –与氧化剂HNO 3之间的反应。在这里,我们将DCA – + HNO 3的计算路径与离子对双氰胺钠与硝酸Na [DCA] + HNO 3的反应坐标进行比较。使用量子力学连续溶剂模型SMD-GIL在气相和溶液中计算焓和自由能。Na [DCA] + HNO 3的壁垒相对于与裸露阴离子的反应,反应显着降低,并产生放热的出口通道以生成NaNO 3和反应性中间体HDCA。这些结果表明,Na [DCA]可能会加速点火反应。
更新日期:2018-02-14
中文翻译:

双氰胺钠与硝酸反应性的理论研究
有必要用更安全的推进剂代替目前的肼燃料,基于双氰胺(DCA –)的系统已成为有希望的替代品,因为它们与某些氧化剂混合时会自动着火。以前对高声反应机理的研究集中在DCA –与氧化剂HNO 3之间的反应。在这里,我们将DCA – + HNO 3的计算路径与离子对双氰胺钠与硝酸Na [DCA] + HNO 3的反应坐标进行比较。使用量子力学连续溶剂模型SMD-GIL在气相和溶液中计算焓和自由能。Na [DCA] + HNO 3的壁垒相对于与裸露阴离子的反应,反应显着降低,并产生放热的出口通道以生成NaNO 3和反应性中间体HDCA。这些结果表明,Na [DCA]可能会加速点火反应。






































 京公网安备 11010802027423号
京公网安备 11010802027423号