当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionic Conductivity of Polyelectrolyte Hydrogels
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acsami.7b15934 Chen-Jung Lee 1 , Haiyan Wu 1 , Yang Hu 2 , Megan Young 2 , Huifeng Wang 2 , Dylan Lynch 2 , Fujian Xu 3 , Hongbo Cong 1 , Gang Cheng 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acsami.7b15934 Chen-Jung Lee 1 , Haiyan Wu 1 , Yang Hu 2 , Megan Young 2 , Huifeng Wang 2 , Dylan Lynch 2 , Fujian Xu 3 , Hongbo Cong 1 , Gang Cheng 2
Affiliation
|
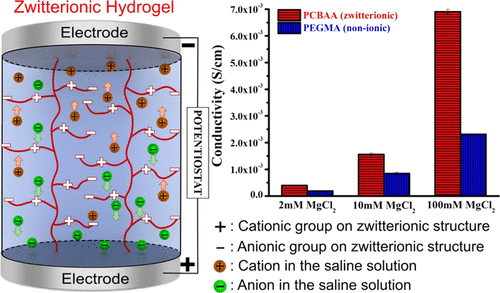
Polyelectrolytes have many important functions in both living organisms and man-made applications. One key property of polyelectrolytes is the ionic conductivity due to their porous networks that allow the transport of water and small molecular solutes. Among polyelectrolytes, zwitterionic polymers have attracted huge attention for applications that involve ion transport in a polyelectrolyte matrix; however, it is still unclear how the functional groups of zwitterionic polymer side chains affect their ion transport and swelling properties. In this study, zwitterionic poly(carboxybetaine acrylamide), poly(2-methacryloyloxyethyl phosphorylcholine), and poly(sulfobetaine methacrylate) hydrogels were synthesized and their ionic conductivity was studied and compared to cationic, anionic, and nonionic hydrogels. The change of the ionic conductivity of zwitterionic and nonionic hydrogels in different saline solutions was investigated in detail. Zwitterionic hydrogels showed much higher ionic conductivity than that of the widely used nonionic poly(ethylene glycol) methyl ether methacrylate hydrogel in all tested solutions. For both cationic and anionic hydrogels, the presence of mobile counterions led to high ionic conductivity in low salt solutions; however, the ionic conductivity of zwitterionic hydrogels surpassed that of cationic and ionic hydrogels in high salt solutions. Cationic and anionic hydrogels showed much higher water content than that of zwitterionic hydrogels in deionized water; however, the cationic hydrogels shrank significantly with increasing saline concentration. This work provides insight into the effects of polyelectrolyte side chains on ion transport. This can guide us in choosing better polyelectrolytes for a broad spectrum of applications, including bioelectronics, neural implants, battery, and so on.
中文翻译:

聚电解质水凝胶的离子电导率
聚电解质在生物体和人造应用中均具有许多重要功能。聚电解质的一个关键特性是离子电导率,这是由于其多孔网络可以输送水和小分子溶质。在聚电解质中,两性离子聚合物在涉及在聚电解质基质中进行离子传输的应用中引起了极大的关注。然而,尚不清楚两性离子聚合物侧链的官能团如何影响其离子传输和溶胀性能。在这项研究中,两性离子聚(羧基甜菜碱丙烯酰胺),聚(2-甲基丙烯酰氧基乙基磷酸基胆碱)和聚(磺基甜菜碱甲基丙烯酸甲酯)水凝胶被合成,并对其离子电导率进行了研究,并与阳离子,阴离子和非离子水凝胶进行了比较。详细研究了两性离子和非离子水凝胶在不同盐溶液中离子电导率的变化。在所有测试溶液中,两性离子水凝胶的离子电导率均比广泛使用的非离子聚(乙二醇)甲基醚甲基丙烯酸酯水凝胶的离子电导率高得多。对于阳离子水凝胶和阴离子水凝胶,流动抗衡离子的存在都会在低盐溶液中导致高离子电导率。然而,在高盐溶液中,两性离子水凝胶的离子电导率超过了阳离子和离子水凝胶的电导率。阳离子和阴离子水凝胶在去离子水中的水含量比两性离子水凝胶高得多。然而,阳离子水凝胶随着盐水浓度的增加而显着收缩。这项工作提供了对聚电解质侧链对离子迁移的影响的见解。这可以指导我们为各种应用选择更好的聚电解质,包括生物电子,神经植入物,电池等。
更新日期:2018-01-31
中文翻译:

聚电解质水凝胶的离子电导率
聚电解质在生物体和人造应用中均具有许多重要功能。聚电解质的一个关键特性是离子电导率,这是由于其多孔网络可以输送水和小分子溶质。在聚电解质中,两性离子聚合物在涉及在聚电解质基质中进行离子传输的应用中引起了极大的关注。然而,尚不清楚两性离子聚合物侧链的官能团如何影响其离子传输和溶胀性能。在这项研究中,两性离子聚(羧基甜菜碱丙烯酰胺),聚(2-甲基丙烯酰氧基乙基磷酸基胆碱)和聚(磺基甜菜碱甲基丙烯酸甲酯)水凝胶被合成,并对其离子电导率进行了研究,并与阳离子,阴离子和非离子水凝胶进行了比较。详细研究了两性离子和非离子水凝胶在不同盐溶液中离子电导率的变化。在所有测试溶液中,两性离子水凝胶的离子电导率均比广泛使用的非离子聚(乙二醇)甲基醚甲基丙烯酸酯水凝胶的离子电导率高得多。对于阳离子水凝胶和阴离子水凝胶,流动抗衡离子的存在都会在低盐溶液中导致高离子电导率。然而,在高盐溶液中,两性离子水凝胶的离子电导率超过了阳离子和离子水凝胶的电导率。阳离子和阴离子水凝胶在去离子水中的水含量比两性离子水凝胶高得多。然而,阳离子水凝胶随着盐水浓度的增加而显着收缩。这项工作提供了对聚电解质侧链对离子迁移的影响的见解。这可以指导我们为各种应用选择更好的聚电解质,包括生物电子,神经植入物,电池等。































 京公网安备 11010802027423号
京公网安备 11010802027423号