当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereo- and Enantioselective Dearomative [3 + 2] Cycloaddition Reaction of 2-Nitrobenzofurans with 3-Isothiocyanato Oxindoles
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acs.orglett.7b03667
Jian-Qiang Zhao 1 , Xiao-Jian Zhou 2, 3 , Yan Zhou 4 , Xiao-Ying Xu 2 , Xiao-Mei Zhang 2 , Wei-Cheng Yuan 2
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-31 00:00:00 , DOI: 10.1021/acs.orglett.7b03667
Jian-Qiang Zhao 1 , Xiao-Jian Zhou 2, 3 , Yan Zhou 4 , Xiao-Ying Xu 2 , Xiao-Mei Zhang 2 , Wei-Cheng Yuan 2
Affiliation
|
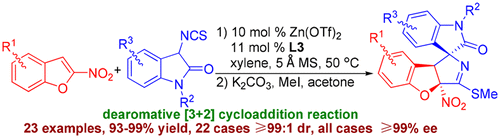
Enantioselective dearomative [3 + 2] cycloaddition reaction of 2-nitrobenzofurans with 3-isothiocyanato oxindoles was developed. The reaction employs a chiral bis(oxazoline)/Zn(OTf)2 catalyst, allowing a practical, straightforward access to structurally diverse spirooxindoles containing a 2,3-dihydrobenzofuran motif and three contiguous stereocenters with excellent diastereo- and enantioselectivities. The synthetic potentials of the method have been demonstrated by the scale-up experiment and transformations of the products. The preliminary mechanism was investigated with experimental observations, nonlinear effects studies, and MS experiments.
中文翻译:

2-硝基苯并呋喃与3-异硫氰酸根合吲哚的非对映和对映选择性脱芳香[3 + 2]环加成反应
开发了2-硝基苯并呋喃与3-异硫氰酸根合吲哚的对映选择性脱芳香性[3 + 2]环加成反应。该反应采用手性双(恶唑啉)/ Zn(OTf)2催化剂,可以实际,直接地获得结构多样的螺硫醇,该螺硫醇含有2,3-二氢苯并呋喃基序和三个连续的立体中心,具有非对映选择性和对映选择性。该方法的合成潜力已通过放大实验和产品转化证明。通过实验观察,非线性效应研究和MS实验研究了初步机理。
更新日期:2018-01-31
中文翻译:

2-硝基苯并呋喃与3-异硫氰酸根合吲哚的非对映和对映选择性脱芳香[3 + 2]环加成反应
开发了2-硝基苯并呋喃与3-异硫氰酸根合吲哚的对映选择性脱芳香性[3 + 2]环加成反应。该反应采用手性双(恶唑啉)/ Zn(OTf)2催化剂,可以实际,直接地获得结构多样的螺硫醇,该螺硫醇含有2,3-二氢苯并呋喃基序和三个连续的立体中心,具有非对映选择性和对映选择性。该方法的合成潜力已通过放大实验和产品转化证明。通过实验观察,非线性效应研究和MS实验研究了初步机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号